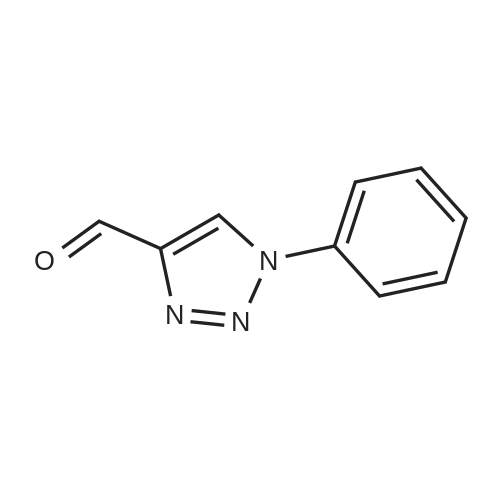
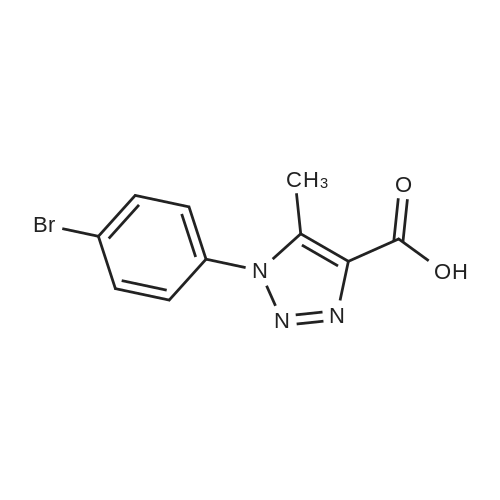
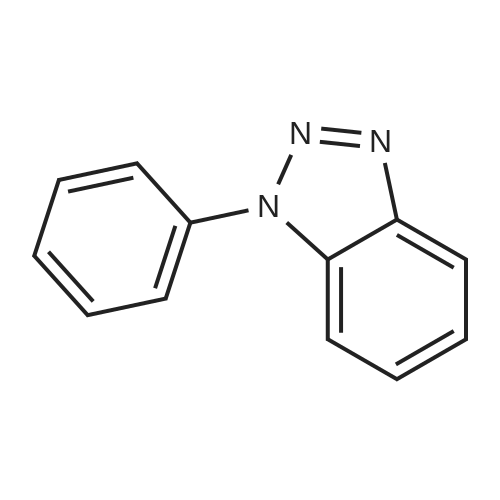
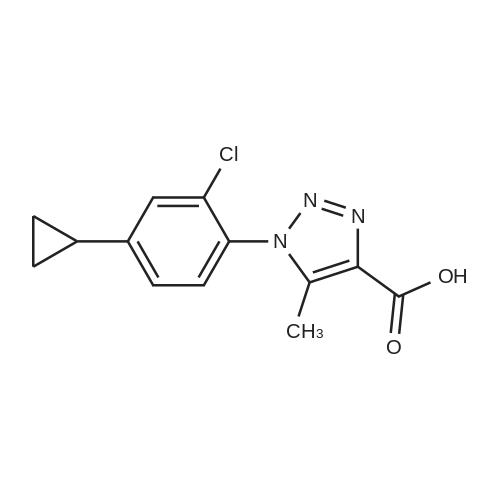
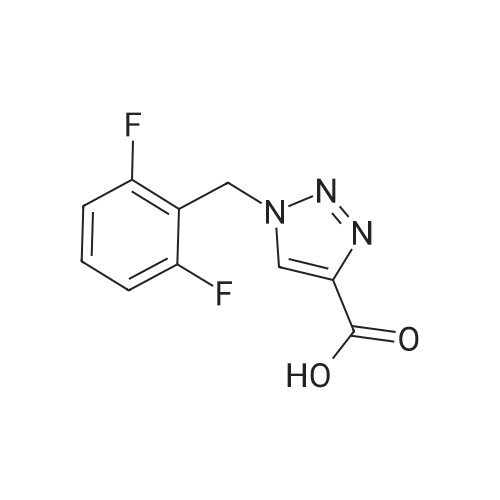
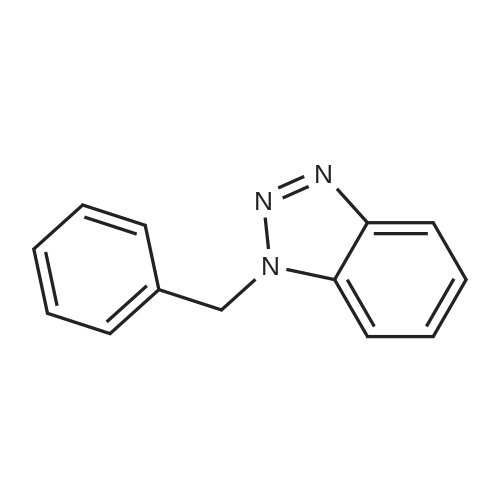
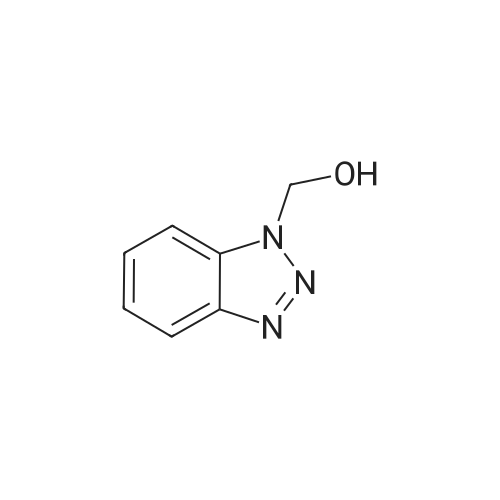
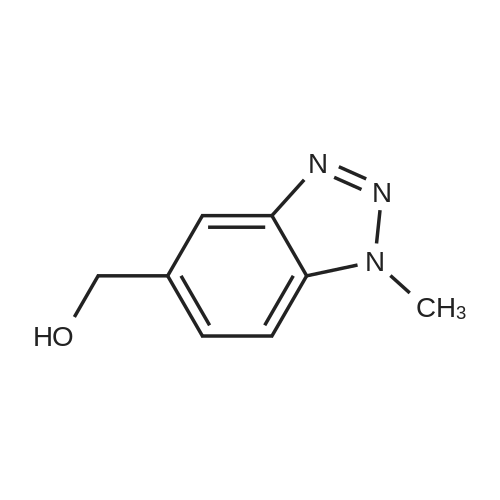
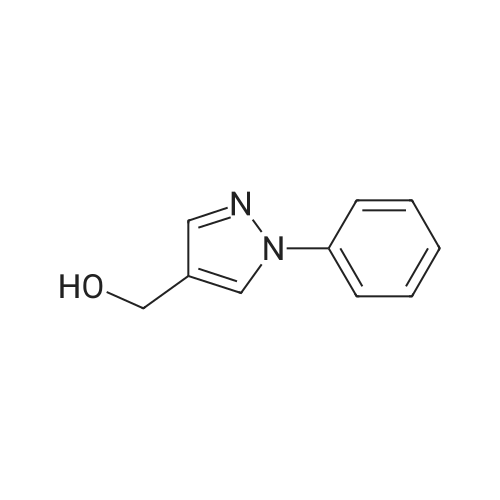
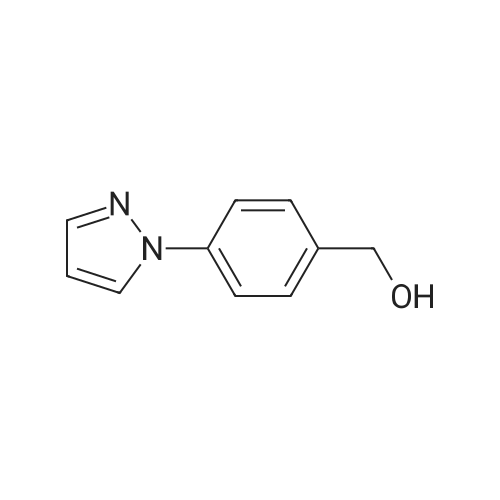
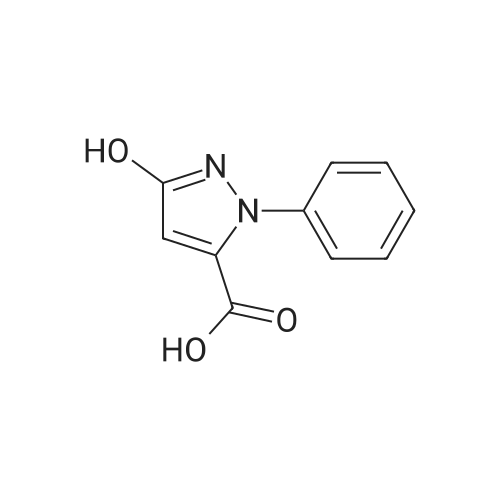
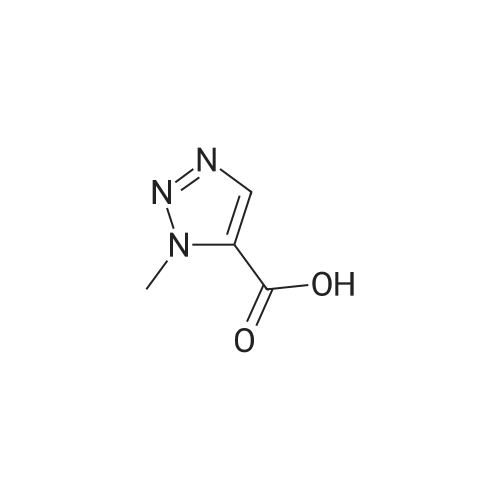
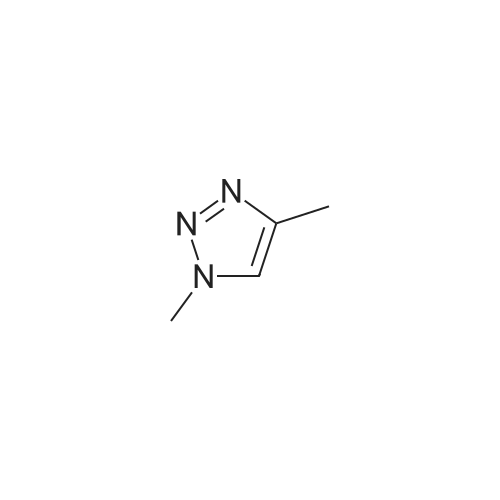
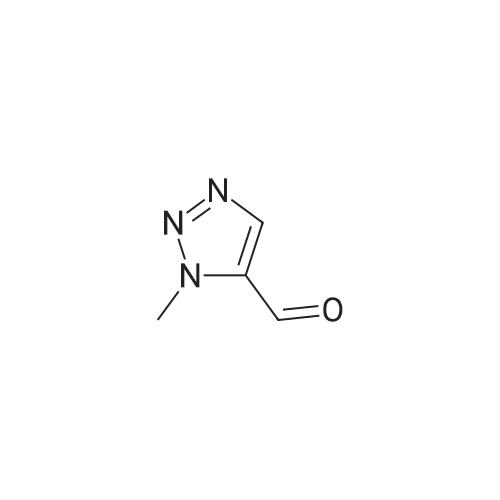
| 89% |
With CuNP(at)hydrotalcite; In ethylene glycol; at 20℃; for 1.75h; |
General procedure: To a mixture of azide 1 (1 mmol, 1 equiv.) and acetylene 2 (1.1 mmol, 1.1 equiv.) in ethylene glycol (3 mL) was added the catalyst (15 mg, 1.87 wt%). The mixture was stirred at room temperature for different time. The progress of the reaction was monitored by TLC. The product was extracted with ethyl acetate and dried over anhydrous Na2SO4. The product was purified by column chromatography over silica gel using n-hexane/ethyl acetate to get the desired product. The products were characterized by 1H & 13C NMR spectroscopy. |
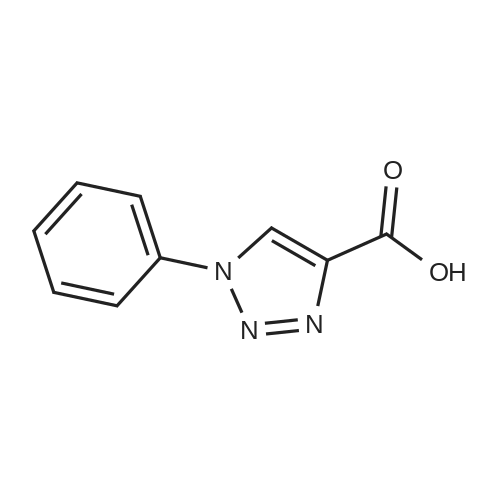
| 88% |
With silver trifluoromethanesulfonate; copper; In acetonitrile; at 20℃; for 3h;Inert atmosphere; |
General procedure: In a typical experiment, alkyne (1mmol) and azide (1 mmol) were dissolved in anhydrous acetonitrile (5 mL) under nitrogen atmosphere. AgOTf (15 mol%) and copper powder (50 mol%) were added to the reaction mixture and stirring continued at r.t. for 3 hr. The reaction mixture was filtered and the filtrate was concentrated under reduced pressure. The product was extracted with DCM. The organic layer was washed with water, dried over sodium sulphate, concentrated and purified by column chromatography using petroleum ether and ethyl acetate as eluent. |
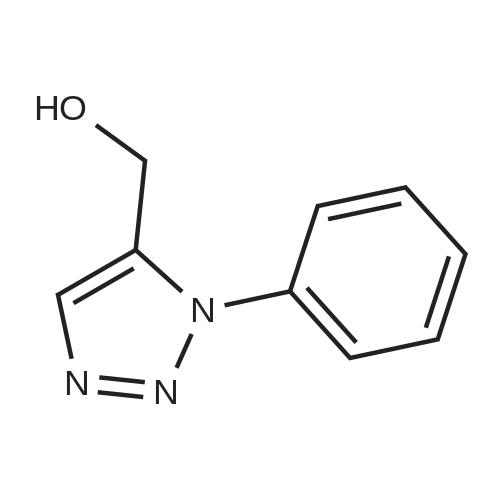
| 87% |
With copper(l) iodide; triethylamine; In water; tert-butyl alcohol; at 20℃; |
General procedure: The corresponding azide (1 mmol) and terminal alkyne (1 mmol or 2 mmol in the reactions with diazides) were dissolved in 5 mL of THF or tert-butyl alcohol. Water was added to the solution until an emulsion began to form, and a cata-lytic amount of copper(I) iodide (1-10 mol %, de-pending on the azide reactivity) was added. In the reac-tions with weakly reactive azides, 0.4 mL (2.8 mmol) of triethylamine as a co-catalyst was added. The mix-ture was stirred at room temperature until the initial azide disappeared (according to the TLC or IR data). The mixture was treated with 15 mL of water and 15 mL of concentrated aqueous ammonia and extracted with methylene chloride (3 × 10 mL). The extract was dried over Na 2 SO 4 , and the solvent was evaporated under reduced pressure. If necessary, the product was purified by recrystallization or column chromatog-raphy. Compounds 3d-3g were obtained as the only products: 3d, yield 95%; 3e, yield 89%; 3f, yield 91%; 3g, yield 67%. |
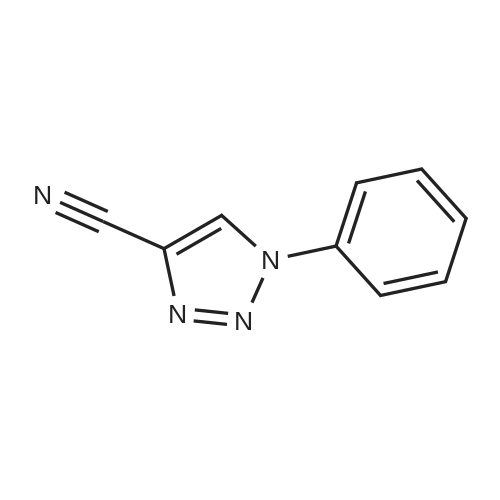
| 70% |
With copper(l) iodide; N,N,N',N'',N'''-pentamethyldiethylenetriamine; In tetrahydrofuran; at 20℃;Sonication; Inert atmosphere; |
General procedure: In a 50 mL two-neck flask under a nitrogen atmosphere, a propargyl alcohol solution (0.28 g, 0.3 mL, 5 mmol) was added with a dissolved organic azide (1.2 equiv, 6 mmol) in THF (8 mL) and the copper catalyst (1 equiv, 5 mmol). The reaction was then put in a ultrasound bath for homogenization, followed by the addition of PMDETA (1.03 g, 1.25 mL, 6 mmol) drop by drop until the starting material was consumed, followed by TLC. The resulting aqueous phase was washed with ethyl acetate, the organic phase was dried with MgSO4, filtered and the solvent was evaporated under vacuum. The crude product was purified by column chromatography using as the eluent a mixture of hexane/ethyl acetate (2/8) (see refPreviewPlaceHolderSupplementary data for reaction time). |
| 63% |
|
To a mixture of DMSO: H2O (9:1) (40mL) solution phenyl azide (4.09g, 34.33mmol, 1.1equiv.) and copper iodide (1.19g, 6.24mmol, 0.2equiv.) were added and stirred for 10min. To this propargyl alcohol (1.75g, 31.21mmol, 1equiv.) was added and stirred additionally for 24h. Upon adding the reaction mixture to ice cold water pale green solid was precipitated. Solvent was filtered off and precipitate was washed with water (5×100mL), acetone (10mL) and dried under vacuo to give 1 as a pale green solid in (3.4g) 63% yield. Mp: 102C (decomposed); IR (KBr): 3381 (nuOH), 2924, 1594, 1011cm-1; 1H NMR (400MHz, CDCl3) delta: 8.01 (s, 1H), 7.69-7.66 (m, 2H), 7.49-7.39 (m, 3H), 4.87 (-CH2-OH) (d, 2H, J=4.4Hz), 3.91 (broad, s, 1H) (-OH); 13C NMR (100MHz, CDCl3) delta: 148.6, 137.0, 129.7, 128.9, 120.5, 120.3, 56.3 (-CH2-OH). |
|
With copper(ll) sulfate pentahydrate; sodium L-ascorbate; In water; tert-butyl alcohol; at 20℃; |
General procedure: To a round-bottom flask equipped with a magnetic stirring bar were added an aromatic azide (0.83mmol), propargyl alcohol (0.75mmol), tert-butanol (0.7mL), copper sulfate pentahydrate (0.04mmol), sodium ascorbate (0.11mmol) and water (0.7mL). The reaction mixture was stirred for 48-72hat room temperature. Next, the mixture was extracted with ethyl acetate and the combined organic extracts were washed with water, dried over anhydrous sodium sulfate, filtered and concentrated in vacuo. The product was purified via silica-gel column chromatography, using gradient mixture of hexane-ethyl acetate, to afford the pure derivatives 12a-d. |
|
With copper diacetate; In water; tert-butyl alcohol; at 20℃; for 18h;Inert atmosphere; |
General procedure: In an argon atmosphere, alkyl/aryl azide (5.4mmol), propargyl alcohol (8mmol) were taken in t-BuOH (5mL) followed by addition of an aqueous Cu(OAc)2 solution (5mol%, 1mL). The reaction was stirred for 18h at room temperature and monitored by TLC. The resulting mixture was diluted with dichloromethane; the organic layer was separated and washed with water. Dried over sodium sulfate and purified by passing through a column packed with silica gel using n-hexane: ethyl acetate (6:4) as solvents. |
|
In water; at 25℃; for 5h; |
General procedure: Phenylboronic acid 1a (1.5 mmol), NaN3 (1.5 mmol), and catalyst(0.03 mmol) were added to a solution of H2O (5 ml). The mixturewas stirred at ambient temperature for 7 h a correspondingtime. The progress was monitored by TLC. After this, phenylacetylene3a (1.0 mmol) was added, and the mixture was stirredat ambient temperature until complete. The reaction mixturewas adjusted to pH 12 with 1 M NaOH and then filtered. The filtercake was washed with EtOAc and THF twice each. The filtrate wasdiluted with water and extracted with EtOAc several times. Theorganic layer was separated, washed with saturated brine, anddried over anhydrous sodium sulfate and the solvent was removedunder vacuum. The crude residue was purified by flash chromatographyon silica gel to give the final product, 4a. |
|
With copper(l) iodide; sodium L-ascorbate; In acetonitrile; at 80℃; for 5h;Sealed tube; |
General procedure: 1-Azido-4-methylbenzenes 1 (0.3 mmol), propargyl alcohol 2 (0.36 mmol), CuI (0.03 mmol),NaAsc (0.06 mmol), and 2 mL solvent were added to a 15 mL pressure tube. The tube was thensealed, and the mixture was stirred at 80 C for 5 hours. After the reaction completed, the abovesystem was added with KMnO4 (0.75 mmol) and Na2CO3 (0.45 mmol), and stirred at 80 C for 8h until the reaction completed. Then, Ag2O (0.03 mmol) and K2S2O7 (0.6 mmol) were added tothe tube and the mixture was conducted at 100 C for 24 h until the transformation finished byTLC analysis. H2O (25 mL) was added to the mixture and the system was extracted with EtOAc (3× 20 mL). The combined organic layer was washed with brine (3 × 5 mL), dried with Na2SO4, andconcentrated under reduced pressure to afford the crude product. Purification by columnchromatography on silica gel with EtOAc-PE (1:3) afforded the desired product 3. |
|
With copper(l) iodide; N-ethyl-N,N-diisopropylamine; In ethanol; at 25℃; for 4h; |
General procedure: Propynyl alcohol (2.2 g, 0.04 mol), cuprous iodide (0.4 g, 2.0 mmol) andN,N-diisopropylethylamine (5.2 g, 0.04 mol) were sequentially added into a stirred solution of intermediate9a-9l(0.04 mol) in absolute ethanol (10 v/w) at 25oCfor 24 h. The insoluble matter removed by filtration, and the filtrate is concentrated. Next the filtrate was poured into water, extracted with dichloromethane, and the combined organic layer was washed with water, dried over anhydrous Na2SO4and evaporated to dryness to give compounds10a-10l. Intermediate10a-10l(0.10 mol) without purification was dissolved in glacial acetic acid (10 v/w), chromium trioxide (2 mL, 0.01 mol) was added dropwise and the mixture was stirred 1 h at 100oC.After cooling to r.t., solvent was removed by concentrate under reduced pressure. The residue was added to water under stirring, the precipitates were collected by filtration and washed with water to obtain compounds11a-11l. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping