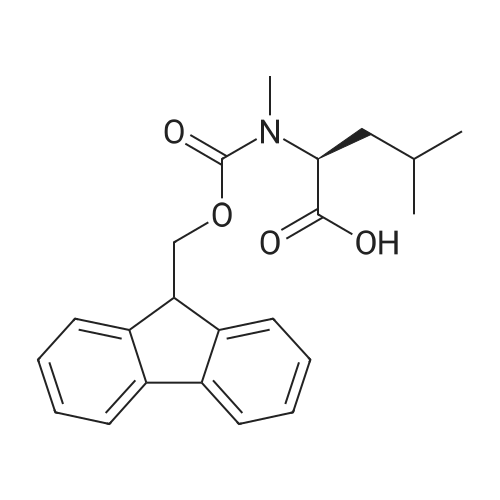
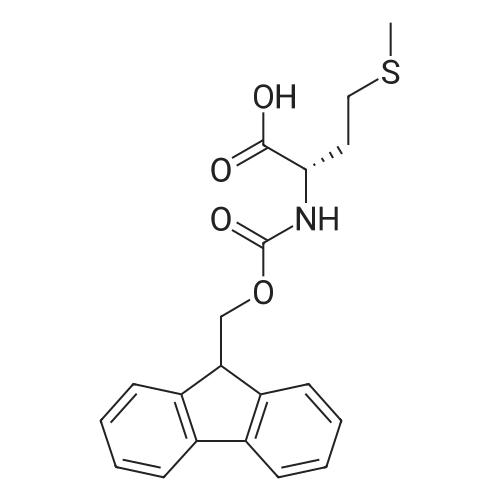
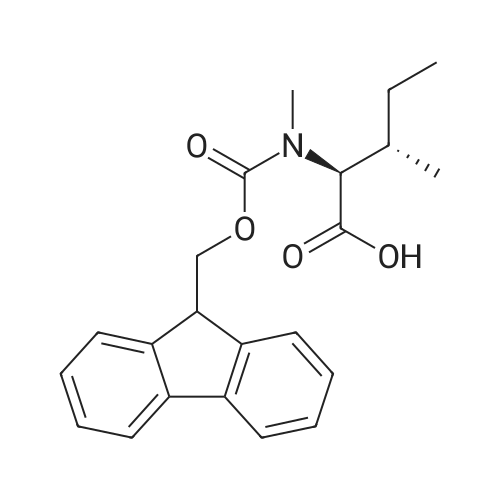
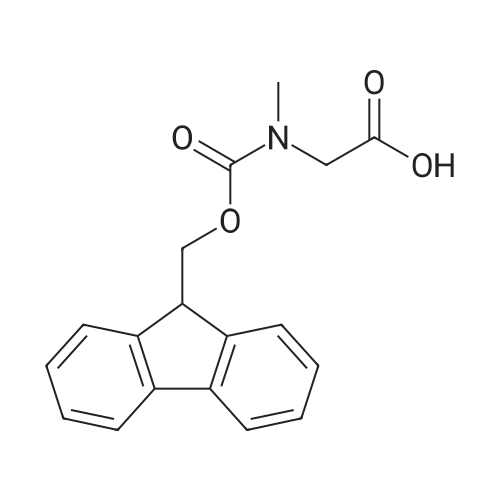
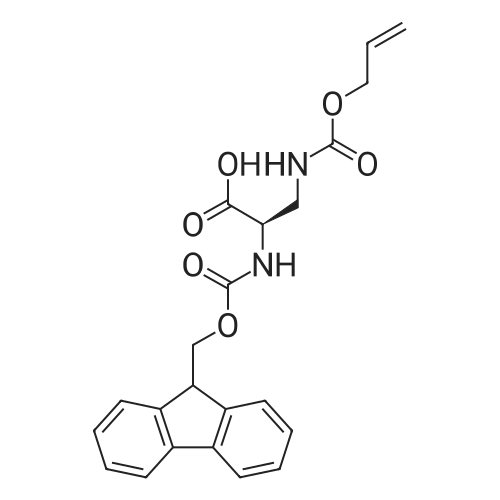
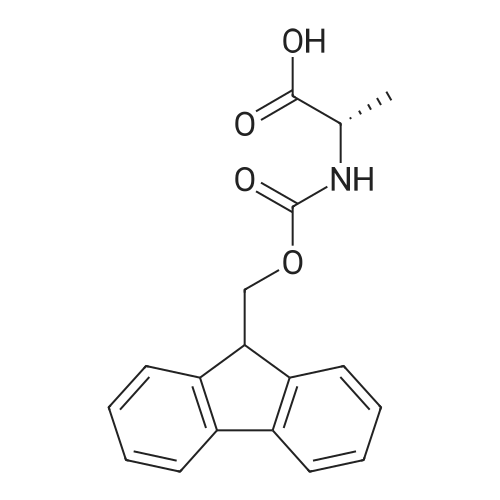
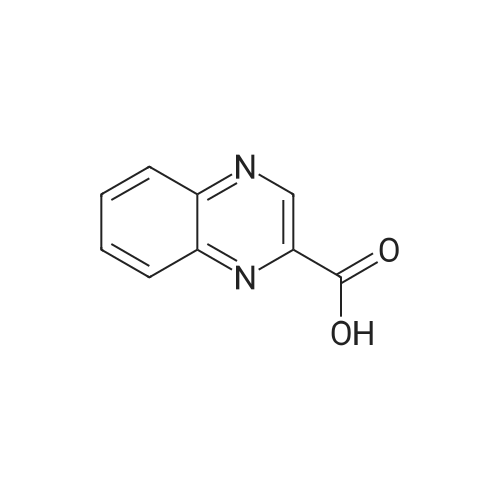
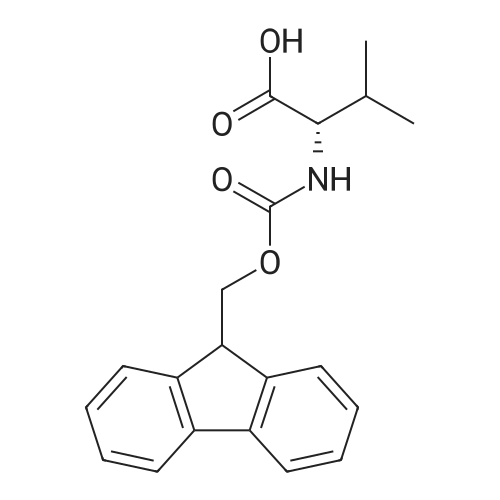
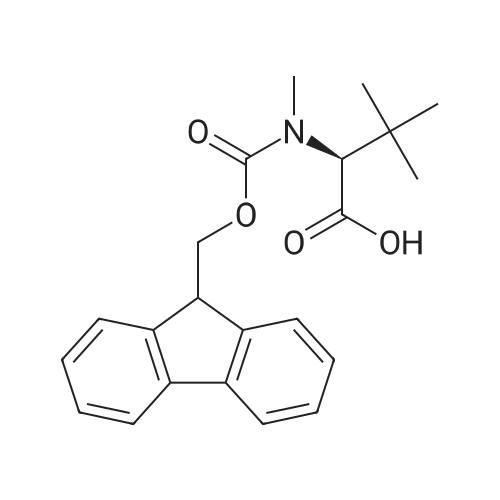
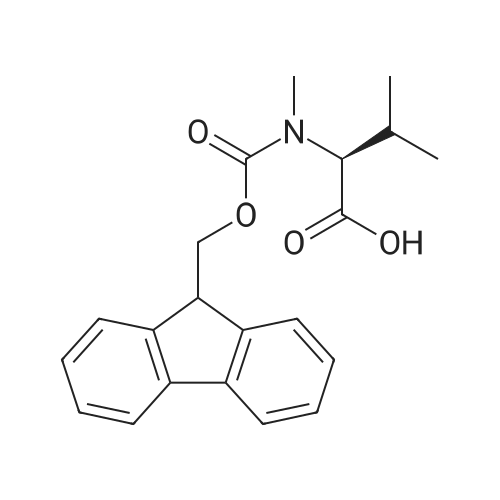
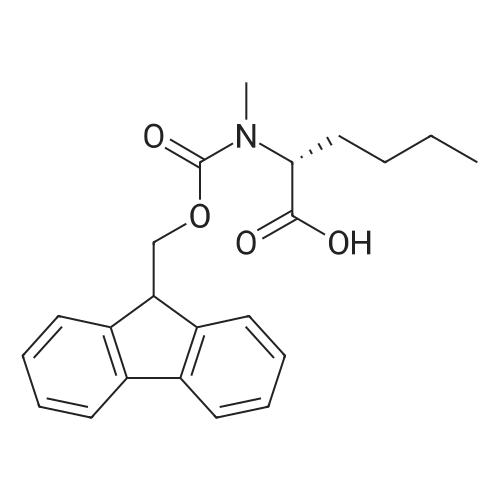
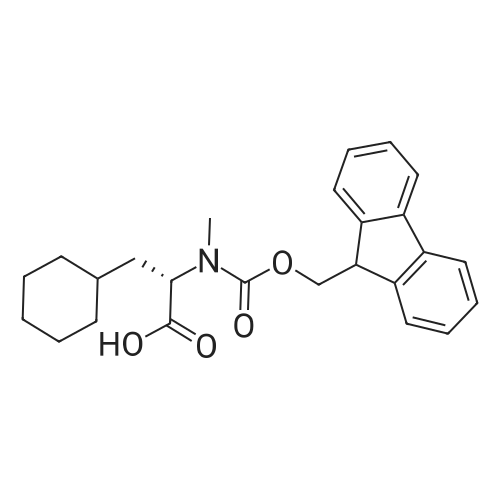
| 98.8 mg |
|
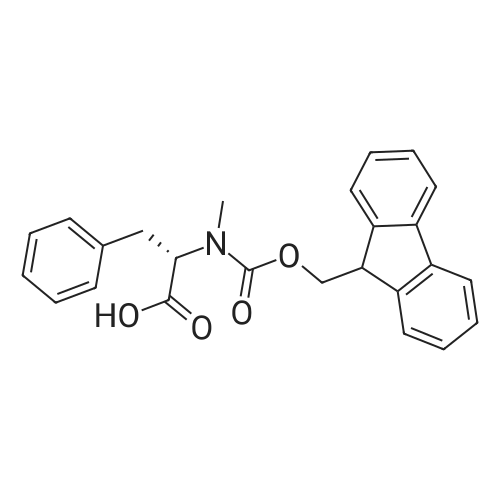
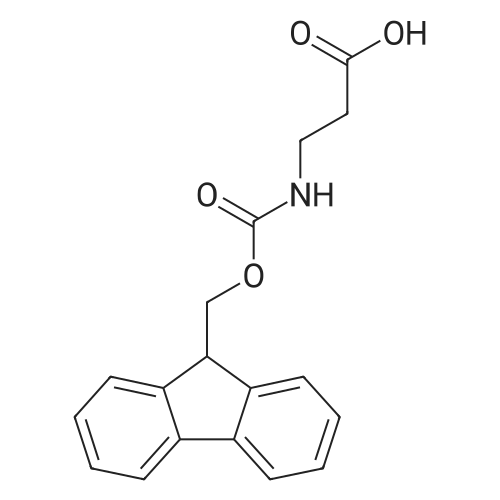
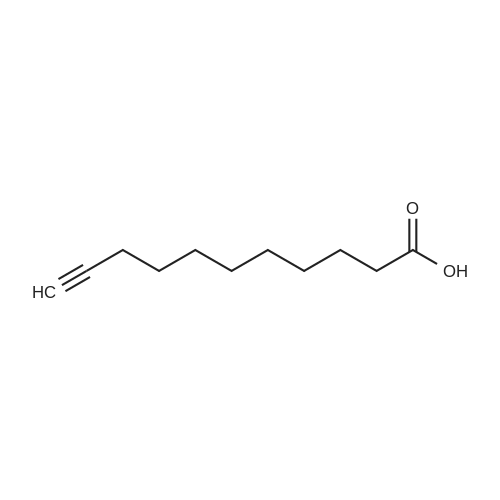
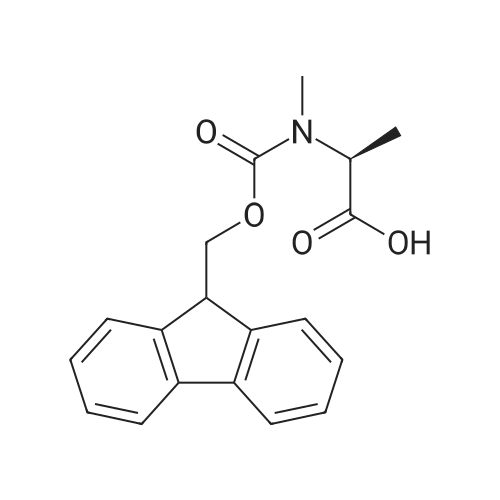
2-Chlorotrityl resin (1.80g, 2.00mmol) was added to dry dichloromethane (10mL) and a solution of Fmoc-l-proline (843mg, 2.50mmol) that was previously treated with dry dichloromethane (10mL) and N,N-diisopropylethylamine (1.71mL, 10.0mmol). The mixture was shaken for 3?4h. Methanol (3mL) was then added and the mixture was shaken for another 10min. The latter step was undertaken in two cycles. The resin was then filtered and washed successively with dichloromethane, dimethylformamide and dichloromethane. The resin was dried in vacuo for 30min by a stream of air to obtain dry Fmoc-propyl resin (57percent). A 1.5mmol portion of dry Fmoc-propyl resin was employed in the synthesis of [2S,3S-Hmp]-aureobasidin L 2. 4.2.6 Fmoc deprotection 1 (0035) Fmoc-peptidyl resin was shaken with 20percent dry piperidine in dry dimethylformamide (20mL) for 30min. The resin was then filtered and washed with dichloromethane, dimethylformamide and dichloromethane successively. The exposed primary amine was tested by the addition of a TNBS test solution while N-methylated secondary amine was tested by adding chloranil test solution to the resin beads. 4.2.7 Fmoc deprotection 2 (0036) Fmoc-depsipeptidyl resin was shaken with dry piperidine/ DBU/ dimethylformamide (2:2:96, 20mL) for 10?20min. The resin was then filtered and washed with dichloromethane, dimethylformamide and dichloromethane successively. The exposed primary amine was tested by the addition of a TNBS test solution while N-methylated secondary amine was tested for by adding chloranil test solution to the resin beads. 4.2.8 HBTU/HOBt-mediated coupling (0037) To the dry depsipeptidyl resin (1.50mmol) having been swollen by dry dichloromethane/ dimethylformamide (1:1, 10mL) was added Fmoc-amino acid (5.00mmol) that was previously treated with O-benzotriazole-N,N,N?,N?-tetramethyl-uronium-hexafluoro-phosphate (1.88g, 5.00mmol), N-hydroxybenzotriazole (671mg, 5.00mmol) and dry N,N-diisopropylethylamine (5.30mL, 15.0mmol) in dry dichloromethane/dimethylformamide (1:1, 10mL). The resin was shaken for 3?24h. The resin was then filtered and washed with dichloromethane, dimethylformamide and dichloromethane successively. To a small portion of dry resin beads was added a TNBS test solution for a non-N-methylated amino acid confirmation or a chloranil test solution for a N-methylated amino acid confirmation. 4.2.9 BTC-mediated coupling (0038) The dry depsipeptidyl resin was swollen with dry N,N-diisopropylethylamine/tetrahydrofuran (1:1, 5.3mL:5.3mL). In a separate vessel, Fmoc-amino acid (5equiv), bis-(trichloromethyl)carbonate (1.65equiv)and dry tetrahydrofuran (20mL) were mixed to give a clear solution. Later, sym-collidine (14.0equiv) was added to that solution to give a white suspension. The suspension was stirred for 1min before it was added to the swollen resin. The resin was shaken for 3?24h. The resin was then filtered and washed with dichloromethane, dimethylformamide and dichloromethane successively. To a small portion of dry resin beads was added a TNBS test solution for a non-N-methylated amino acid confirmation or a chloranil test solution for a N-methylated amino acid confirmation. 4.2.10 HATU/HOAt-mediated coupling (0039) To the dry depsipeptidyl resin (1.50mmol) having been swollen by dry dichloromethane/ dimethylformamide (1:1, 10mL) was added Fmoc-amino acid (5.00mmol) that was previously treated with 2-(1H-7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate methanaminium (1.68g, 5.00mmol), 1-hydroxy-7-azabenzotriazole (681mg, 5.00mmol) and N,N-diisopropylethylamine (5.3mL, 15.0mmol) in dry dichloromethane/dimethylformamide (1:1, 10mL). The resin was shaken for 3?24h. The resin was then filtered and washed withdichloromethane, dimethylformamide and dichloromethane successively. To a small portion of dry resin beads was addeda TNBStest solution for a non-N-methylated amino acid confirmationora chloranil test solution for a N-methylated amino acid confirmation. 4.2.11 Resin cleavage (0040) To the depsinonapeptidyl-trichlorotrityl resin 13 (1.50mmol) was added a cleavage cocktail of trifluoroacetic acid/dry dichloromethane (1:19, 10mL). The yellow resin turned bright red. The resin was then shaken for 1h and then filtered. The resin was washed subsequently with further cleavage cocktail (10mL×2) and dry dichloromethane (10mL×2). The combined solution was evaporated and the resulting residue was dissolved in acetonitrile/water (1:1, 10mL). The solution was then freeze-dried to obtain a brown solid (1.30g). The crude solution (20muL, 1mg/1mL in 50percent acetonitrile in water) was subjected to analytical RP-HPLC on Apollo 5mu, C18 column (250mm×4.6mm), 20percent?90percent acetonitrile in water over 20min, flow rate 1mL/min, 40°Cand monitored at 240nm. There were three major peaks in the crude product, which was further purified by semi-preparative RP-HPLC on an Apollo 5mu, C18 column (250mm×10mm) using 30percent?70percent acetonitrile in water over 6... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping