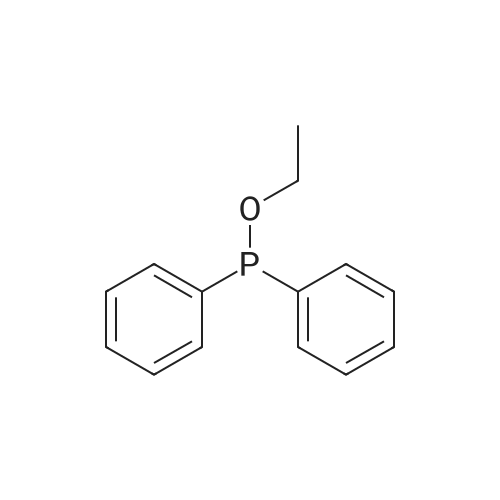
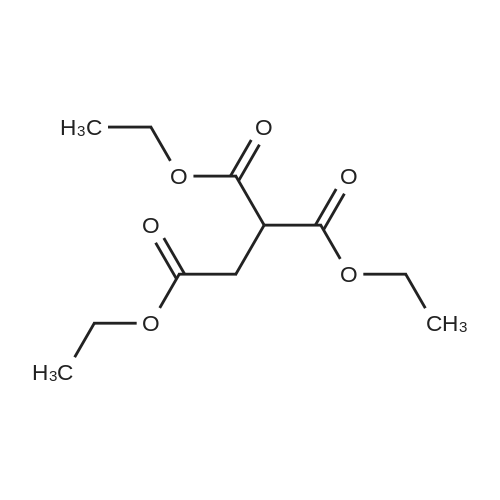
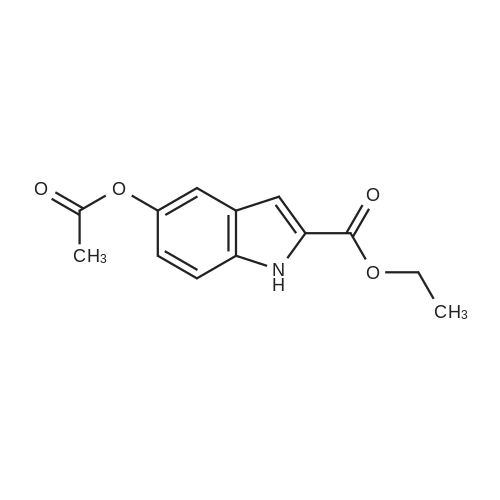
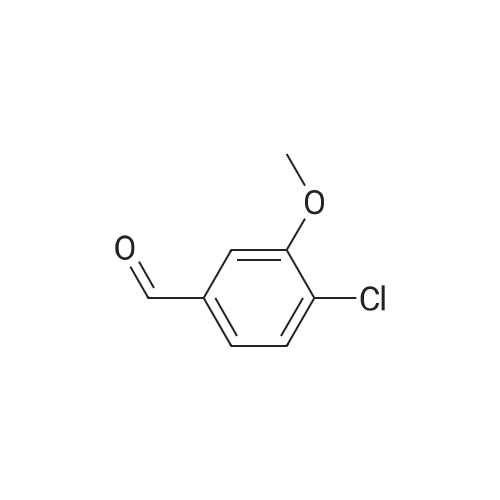
| 92% |
With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile); In tetrachloromethane; for 3h;Heating / reflux; |
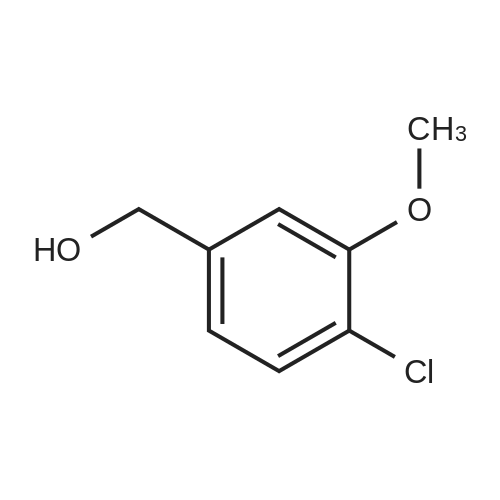
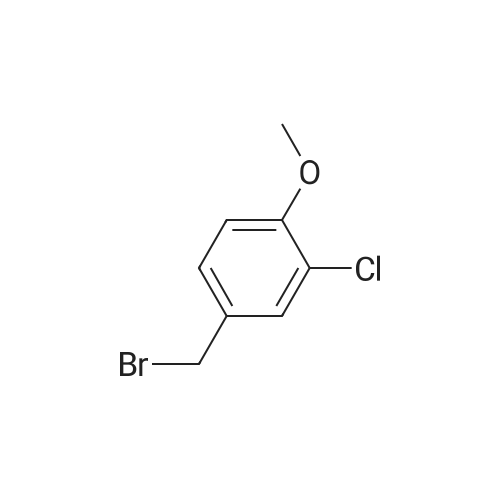
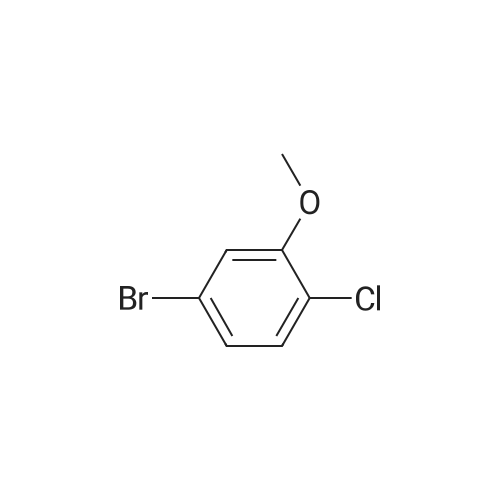
To a solution of 1-chloro-2-methoxy-4-methyl-benzene (50 g, 0.32 mol) in CCl4 (350 mL) was added NBS (57 g, 0.32 mol) and AIBN (10 g, 60 mmol). The mixture was heated at reflux for 3 hours. The solvent was evaporated under vacuum and the residue was purified by column chromatography on silica gel (petroleum ether/ethyl acetate=20:1) to give 4-bromomethyl-1-chloro-2-methoxy-benzene (69 g, 92%). 1H NMR (400 MHz, CDCl3) delta 7.33-7.31 (m, 1H), 6.95-6.91 (m, 2H), 4.46 (s, 2H), 3.92 (s, 3H). |
| 92% |
With N-Bromosuccinimide; azobisisobutyronitrile; In tetrachloromethane; |
4-Bromomethyl-1-chloro-2-methoxy-benzene To a solution of 1-chloro-2-methoxy-4-methyl-benzene (50 g, 0.32 mol) in CCl4 (350 mL) was added NBS (57 g, 0.32 mol) and AIBN (10 g, 60 mmol). The mixture was heated at reflux for 3 hours. The solvent was evaporated under vacuum and the residue was purified by column chromatography on silica gel (petroleum ether/ethyl acetate=20:1) to give 4-bromomethyl-1-chloro-2-methoxy-benzene (69 g, 92%). 1H NMR (400 MHz, CDCl3) delta 7.33-7.31 (m, 1H), 6.95-6.91 (m, 2H), 4.46 (s, 2H), 3.92 (s, 3H). |
| 92% |
With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile); In tetrachloromethane; for 3h;Reflux; |
4-Bromomethyl-1-chloro-2-methoxy-benzene To a solution of 1-chloro-2-methoxy-4-methyl-benzene (50 g, 0.32 mol) in CCl4 (350 mL) was added NBS (57 g, 0.32 mol) and AIBN (10 g, 60 mmol). The mixture was heated at reflux for 3 hours. The solvent was evaporated under vacuum and the residue was purified by column chromatography on silica gel (petroleum ether/ethyl acetate=20:1) to give 4-bromomethyl-1-chloro-2-methoxy-benzene (69 g, 92%). 1H NMR (400 MHz, CDCl3) delta 7.33-7.31 (m, 1H), 6.95-6.91 (m, 2H), 4.46 (s, 2H), 3.92 (s, 3H). |
| 78% |
With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile); In tetrachloromethane; for 3h;Reflux; |
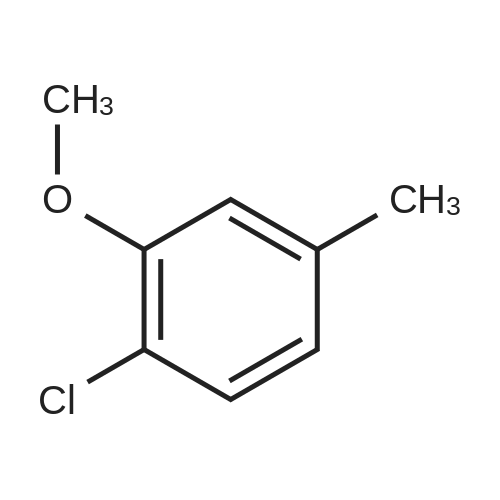
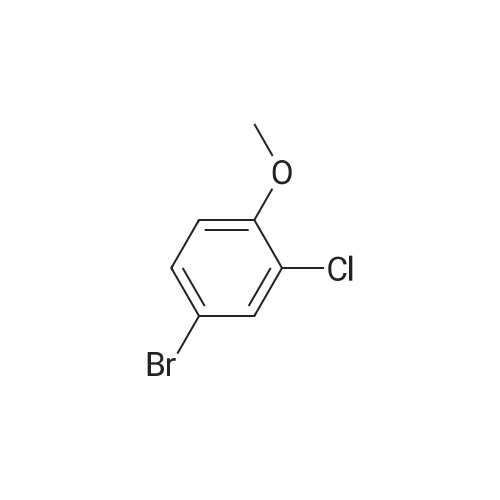
To a stirred solution of 2-chloro-5-methylanisole (16-1, 20.0 g, 127.71 mol) in CCl4 (200 mL) was added AIBN (4.19 g, 25.54 mol) and NBS (22.7 g, 127.7 mol). The resulting reaction mixture was heated under reflux for 3 hours. Then, the reaction mixture was cooled, washed with a 1N HCl aqueous solution, NaHCO3 saturated aqueous solution and brine. The organic layer was dried over Na2SO4, filtered and concentrated under reduced pressure to give 22g (yield = 78%) of a light yellow solid corresponding to 2-chloro-5-(bromomethyl)anisole (16-2). 1H NMR (400 MHz, CDCl3) |
|
With N-Bromosuccinimide; dibenzoyl peroxide; In 1,2-dichloro-ethane; for 3h;Heating / reflux; |
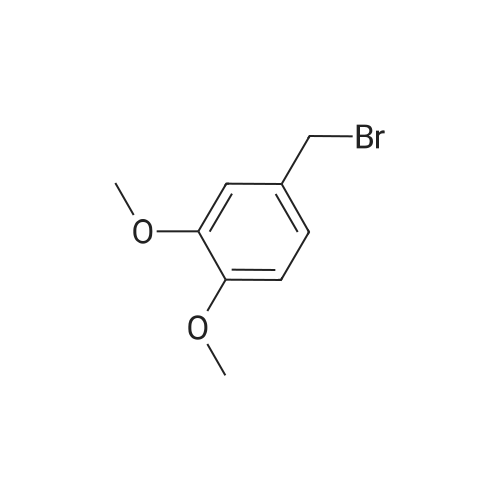
A solution of 4-chloro-3-methoxytoluene (36; 0.5 g; 3.2 mmol), NBS (0.57 g; 3.2 mmol) and benzoyl peroxide (0.031 g; 0.13 mmol) and 32 mL of DCE were heated at reflux for 3 h. The reaction mixture was cooled, diluted with CH2Cl2 and washed with water and brine. The organic extract was dried, filtered and evaporated to yield the bromomethyl compound 38b which was used without further purification. |
|
With N-Bromosuccinimide; dibenzoyl peroxide; In ethyl acetate; for 3h;Irradiation with halogen lamp; |
(i) 4-(BromomethyI)-l-chIoro-2-methoxybenzene 2-Chloro-5-methylrhohenol (20 g), K2CO3 (30 g), acetone (200 ml) and methyl iodide (9.4 ml) were charged to a flask and stirred for 24 h. The solvent was removed under reduced pressure and the residue partitioned between ether and water. The organics were separated, washed with 2 M sodium hydroxide, water, dried (MgSO4) and evaporated under reduced pressure. The residue was dissolved in EtOAc, then NBS (25 g) and benzoyl peroxide (0.5 g) was added and the reaction mixture irradiated with a halogen lamp for 3 h. The solvent was removed under reduced pressure and the residue was purified by flash column chromatography(eluent isohexane) to give the subtitle compound (30 g) used directly without further purification or characterisation. |
|
With N-Bromosuccinimide; dibenzoyl peroxide; In ethyl acetate; for 3h;Irradiation; |
(i) 4-(Bromomethyl)-1-chloro-2-methoxybenzene 2-Chloro-5-methylphenol (20 g), K2CO3 (30 g), acetone (200 ml) and methyl iodide (9.4 ml) were charged to a flask and stirred for 24 h. The solvent was evaporated under reduced pressure and the residue partitioned between ether and water. The organics were separated, washed with 2 M NaOH then water, dried (MgSO4) and evaporated under reduced pressure. The residue was dissolved in ethyl acetate, then NBS (25 g) and benzoyl peroxide (0.5 g) were added and the reaction mixture irradiated with a halogen lamp for 3 h. The solvent was evaporated under reduced pressure and the residue was purified by flash column chromatography (eluent isohexane) to give the subtitle compound (30 g) used directly without further purification or characterisation. |
|
With N-Bromosuccinimide; dibenzoyl peroxide; In 1,2-dichloro-ethane; for 3h;Heating / reflux; |
A solution of 4-chloro-3-methoxytoluene (9a; 0.5 g; 3.2 mmol), NBS (0.57 g; 3.2 mmol) and benzoyl peroxide (0.031 g; 0.13 mmol) and 32 mL of DCE were heated at reflux for 3 h. The reaction mixture was cooled, diluted with CH2Cl2 and washed with water and brine. The organic extract was dried filtered and evaporated to yield the bromomethyl compound 9b which was used without further purification. |
|
With N-Bromosuccinimide; dibenzoyl peroxide; In 1,2-dichloro-ethane; for 3h;Heating / reflux; |
A solution of 4-chloro-3-methoxytoluene (9a; 0.5 g; 3.2 mmol), NBS (0.57 g; 3.2 mmol) and benzoyl peroxide (0.031 g; 0.13 mmol) and 32 mL of DCE were heated at reflux for 3 h. The reaction mixture was cooled, diluted with CH2Cl2 and washed with water and brine. The organic extract was dried filtered and evaporated to yield the bromomethyl compound 9b which was used without further purification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +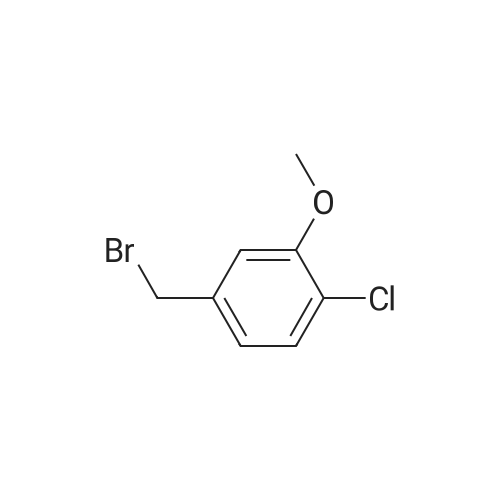

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping