| 94% |
With palladium on activated charcoal; hydrogen; In methanol; at 16 - 19℃; under 1551.49 Torr; for 18h; |
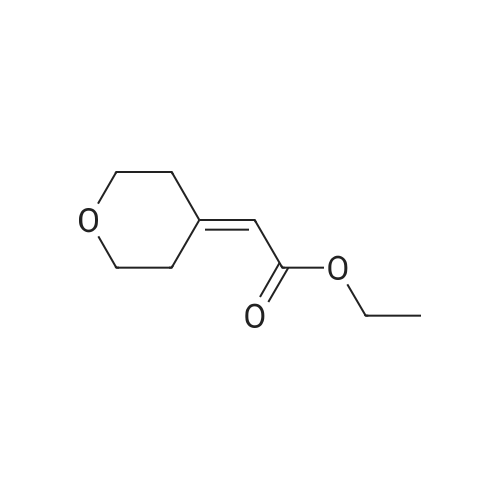
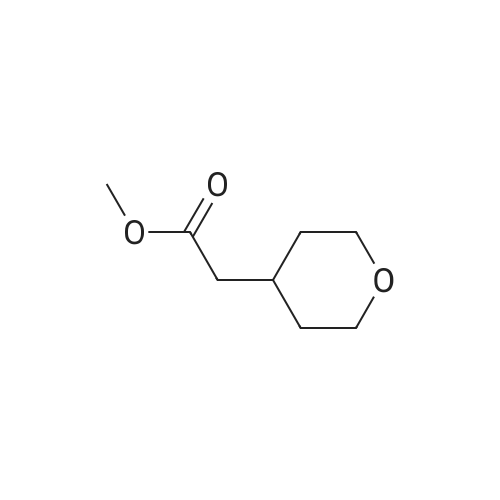
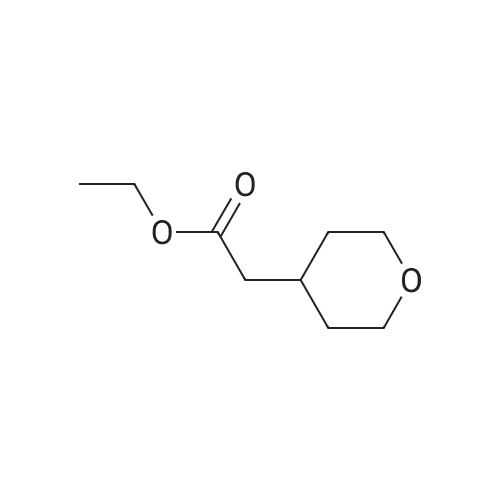
A mixture of ethyl 2-(dihydro-2H-pyran-4(3H)-ylidene) acetate (21 g, 123 mmol) and dry Pd/C (2.5 g) in methanol (300 mE) was stirred at 16-19 C. for 18 h under H2 (30 psi). TEC (petroleum ether:ethyl acetate=3:1) showed no starting material remaining. The mixture was filtered and the filtrate was concentrated under reduced pressure to give crude ethyl5 2-(tetrahydro-2H-pyran-4-yl)acetate (20 g, 94%) as an oil, which was used for the next step directly without further purification. ?H NMR (CDC13, 400 MHz): oe 4.11-4.15 (q, J=7.2 Hz, 2H), 3.93-3.95 (d, J=10.8 Hz, 2H), 3.37-3.43 (t, J=11.6 Hz, 2H), 2.23-2.25 (d, J=6.8 Hz, 2H), 1.99-2.03 (m,10 1H), 1.62-1.65 (m, 2H), 1.32-1.36 (m, 2H), 1.24-1.27 (t, J=7.2 Hz, 3H). |
| 84% |
With sodium tetrahydroborate; nickel dichloride; In methanol; at 0℃; for 0.5h; |
To a solution of ethyl 2-(tetrahydro-4H-pyran-4-ylidene)acetate (0.4 g, 2.33 mmol) in methanol (5 ml) was added NaBH4 (0.198 g, 5.29 mmol) portionwise at 0 C. After 30 mm of stirring at 0 C NiC12 (0.12 g, 0.505 mmol) was added slowly. After stirring for 45mm the reaction solvent was evaporated under reduced pressure and diluted with water (10 ml) and the product was extracted with ethyl acetate (3x 10 ml). The combined organic layer was concentrated under reduced pressure to afford the title compound (84%). ?H NMR (400MHz, CDC13) & 4.18-4.13 (q, 1=7.2Hz, 2H), 3.99-3.95 (m, 2H), 3.46-3.40 (m, 2H), 2.27-2.25 (m, 2H), 2.10-1.98 (m, 1H), 1.64 (s, 2H), 1.41-1.23 (m, 5H). |
| 78% |
With palladium 10% on activated carbon; ammonium formate; In methanol; at 70℃; for 15h; |
To a mixture composed of ethyl 2-(2H-pyran-4(3H,5H,6H)-ylidene)acetate (10 g, 58.75 mmol) and ammonium formate (37 g, 587.54 mmol) in methanol (150 ml) was added 10% Pd-carbon (1.0 g) at room temperature, and the mixture was stirred at 70 C for 15 hr. The reaction mixture was allowed to cool to room temperature, and the catalyst was collected by filtration through Celite pad and was washed with methanol. The filtrate was concentrated under reduced pressure, and the residue was chromatographed on silica gel column (hexane:ethyl acetate = 94:6) to give the title compound as an oil (7.9 g, yield 78%). 1H-NMR (400 MHz, CDCl3): delta (ppm) 4.13 (q, J = 7.2 Hz, 2H ), 3.97-3.93 (m, 2H), 3.44-3.38 (m, 2H), 2.24 (d, J = 7.2 H z, 2H), 2.08-1.92 (m, 1H) 1.66-1.62 (m, 2H), 1.40-1.29 (m, 2H), 1.26 (t, J = 7.2 Hz, 3H); MS (ESI): m/z 172 (M+). |
|
With hydrogen;palladium 10% on activated carbon; In methanol; at 25℃; under 1551.49 Torr; for 20h; |
A mixture of (tetrahydro-pyran-4-ylidene)-acetic acid ethyl ester (27 g, 159 mmol) and 10% palladium on activated carbon (3 g) in methanol (300 mL) was stirred at 25 C. under 30 psi of hydrogen for 20 h. The catalyst was filtered off, washed with ethyl acetate and concentrated in vacuo to afford (tetrahydro-pyran-4-yl)-acetic acid ethyl ester (25 g, 91%) as a colorless oil which was used in the next step without purification. |
|
With palladium on activated charcoal; hydrogen; In methanol; at 16 - 19℃; under 1551.49 Torr; for 18h; |
[00105] A mixture of ethyl 2-(dihydro-2H-pyran-4(3H)-ylidene)acetate (21 g, 123 mmol) and dry Pd/C (2.5 g) in methanol (300 mL) was stirred at 16-19 C for 18 h under H2 (30 psi). TLC (petroleum ether: ethyl acetate = 3: 1) showed no starting material remaining. The mixture was filtered and the filtrate was concentrated under reduced pressure to give crude ethyl 2-(tetrahydro-2H-pyran-4-yl)acetate (20 g, 94%) as an oil, which was used for the next step directly without further purification. 1H NMR (CDC13, 400 MHz): delta 4.11-4.15 (q, J = 7.2 Hz, 2H), 3.93-3.95 (d, J = 10.8 Hz, 2H), 3.37-3.43 (t, J = 11.6 Hz, 2H), 2.23-2.25 (d, J = 6.8 Hz, 2H), 1.99-2.03 (m, 1H), 1.62-1.65 (m, 2H), 1.32-1.36 (m, 2H), 1.24-1.27 (t, J = 7.2 Hz, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping