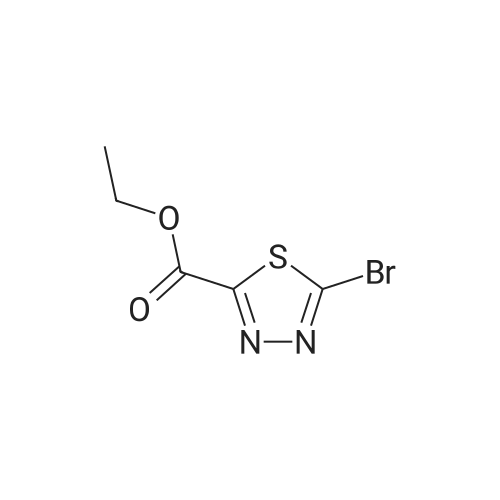
| 74% |
With tert.-butylnitrite; copper(ll) bromide; In acetonitrile; at 20 - 60℃; for 0.666667h; |
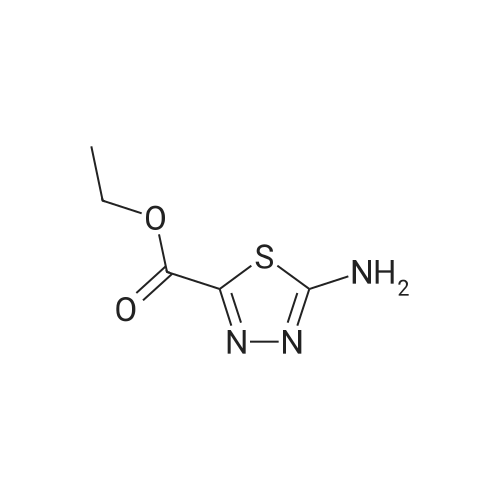
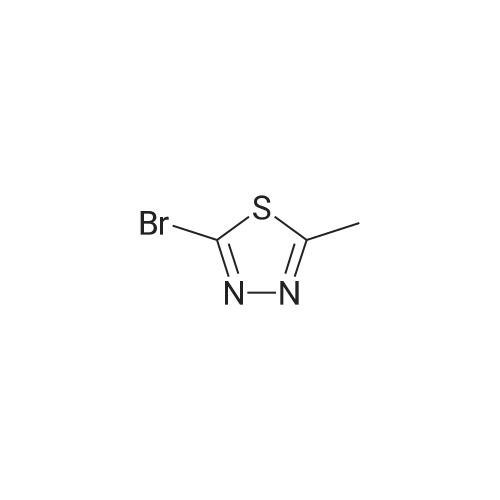
Step 2: ethyl 5-bromo-l,3,4-thiadiazole-2-carboxylate ter-BuONOTo a stirred solution of ethyl 5-amino-l,3,4-thiadiazole-2-carboxylate (3.1 g, 17.8 mmol) in acetonitrile (50 mL) at room temperature was added copper (II) bromide (7.95 g, 35.6 mmol) and the mixture was stirred for 20 min. Tertiary butyl nitrite (3.67 g, 35.63 mmol) was then added drop wise for 10 min, and the reaction mixture was heated to 60°C for 30 min. The reaction mixture was concentrated under reduced pressure, diluted with water (300 mL), and then extracted with ethyl acetate (500 m:). The organic layer was separated and dried over anhydrous sodium sulphate, and evaporated to afford ethyl 5-bromo-l,3,4-thiadiazole-2-carboxylate as a brown solid (3.0 g, 74percent yield). XH NMR (400 MHz, CDC13): ? 4.53-4.51 (m, 2H), 1.45-1.43 (m, 3H); LC-MS m/z calcd for [M+H]+ 238.92, found 238.9. |
|
With tert.-butylnitrite; copper(ll) bromide; In acetonitrile; at 20 - 60℃; for 1.08333h; |
Step 1: Ethyl 5-bromo-1,3,4-thiadiazole-2-carboxylate To a suspension of <strong>[64837-53-2]ethyl 5-amino-1,3,4-thiadiazole-2-carboxylate</strong> (10 g, 58 mmol) in CH3CN (180 mL) was added CuBr2 (25.7 g, 115 mmol). The mixture turned dark green and was further stirred for 15 min at room temperature. t-BuONO, 90percent (13.8 mL, 115 mmol) was added dropwise over 15-20 min. The mixture became slightly warm and gas was evolved after 5 min and then throughout the addition. After completion of the addition and gas evolution subsided, the mixture was heated at 60° C. for 30 min. Solvent was then evaporated in vacuo. Water and EtOAc were added and the mixture was agitated in the flask until the dark green color disappeared. The organic phase became light brown and the aqueous was green with insoluble material. The whole mixture was filtered through celite and washed with EtOAc. The EtOAc layer was separated, washed with dilute brine solution, dried (Na2SO4) and concentrated to give the title compound. 1H NMR (400 MHz, acetone-d6): delta4.52 (q, 2H), 1.43 (t, 3H). |
|
|
To a suspension of ethyl 5-amino-l,3,4- thiadiazole-2-carboxylate in CH3CN (0.32 M) was added CuBr2 (2 equiv). The mixture turned dark green and was stirred for 15 min at room temperature. t-BuONO, 90percent (2 equiv) was added dropwise over 15 - 20 min. The mixture became slightly warm and gas evolved after about 5 min and then throughout the addition. After completion of the addition and after gas evolution subsided, the mixture was heated at 60 °C for 30 min. Solvent was then evaporated in vacuo. Water and EtOAc were added and the mixture was stirred until the dark green color disappeared. The organic phase became light brown and the aqueous phase was green with insoluble material. The entire mixture was filtered through Celite.(TM). and washed with EtOAc. The EtOAc layer was separated, washed with diluted brine, dried (Na2SO4) and concentrated to give the title compound. lH NMR (400 MHz, acetone-^): delta 4.52 (q, 2H), 1.43 (t, 3H). |
|
|
To a suspension of ethyl 5-amino- l,3,4-thiadiazole-2-carboxylate in CH3CN (0.32 M) was added CuBr2 (2 equiv). The mixture turned dark green and was stirred for 15 min at room temperature. Then /-BuONO (2 equiv, 90percent) was added dropwise over 15 - 20 min. The mixture became slightly warm and gas evolved after about 5 min and then throughout the addition. After completion of the addition and after gas evolution subsided, the mixture was heated at 60 °C for 30 min. Solvent was then evaporated in vacuo. Water and EtOAc were added and the mixture was stirred until the dark green color disappeared. The mixture was filtered through Celite.(TM). and washed with EtOAc. The EtOAc layer was separated, washed with diluted brine, dried (Na2SO4) and concentrated to give the title compound. lH NMR (400 MHz, acetone-t/6): delta 4.52 (q, 2H), 1.43 (t, 3H). |
|
|
Step 1 Ethyl 5-bromo-l ,3,4-thiadiazole-2-carboxylateTo a suspension of ethyl 5-ammo-l ,3,4-thiadiazole-2-carboxylate in CH3CN (0 32 M) was added CuBr2 (2 equiv) The mixture turned dark green and was stirred for 15 mm at room temperature ^-BuONO, 90percent (2 equiv) was added dropwise over 15 - 20 mm The mixture became slightly warm and gas was evolved after about 5 min and then throughout the addition After completion of the addition and gas evolution subsided, the mixture was heated at 60 0C for 30 mm Solvent was then evaporated under diminished pressure Water and EtOAc were added and the mixture was stirred until the dark green color disappeared The organic phase became light brown and the aqueous phase was green with insoluble material The entire mixture was filtered through celite and washed with EtOAc The EtOAc layer was separated, washed with diluted bpine, dpied (Na2SO4) and concentrated to give the title compound lH NMR (400 MHz, acetone-d) delta 4 52 (q, 2H), 1 43 (t, 3H) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping