| 58.1% |
With acetic acid; at 100℃; for 14.0h; |
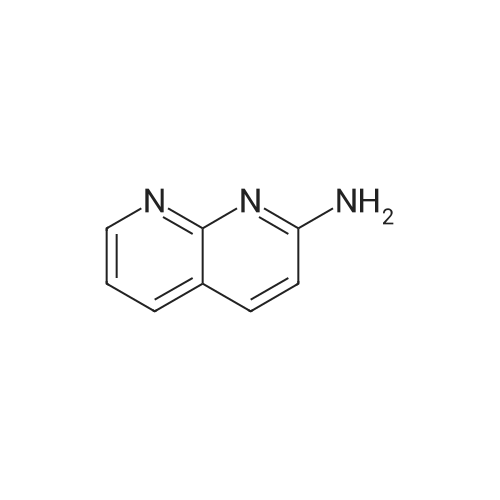
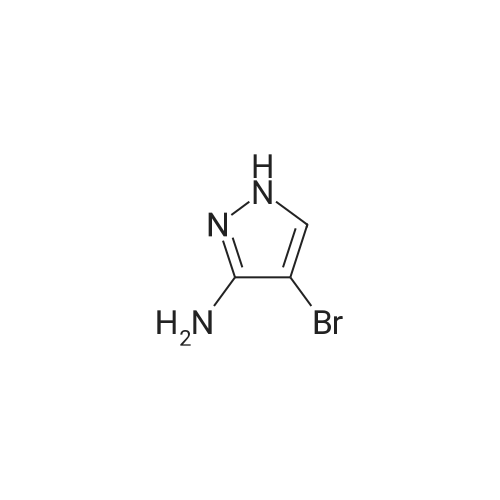
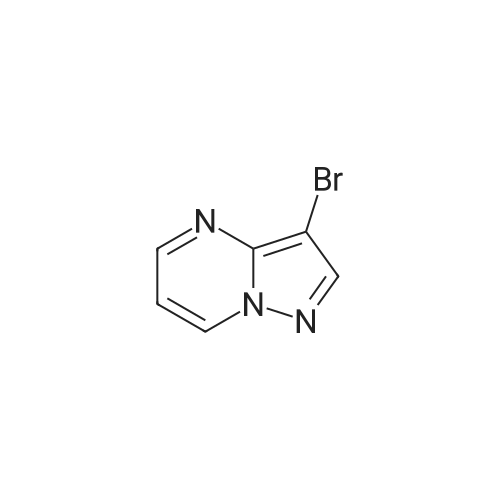
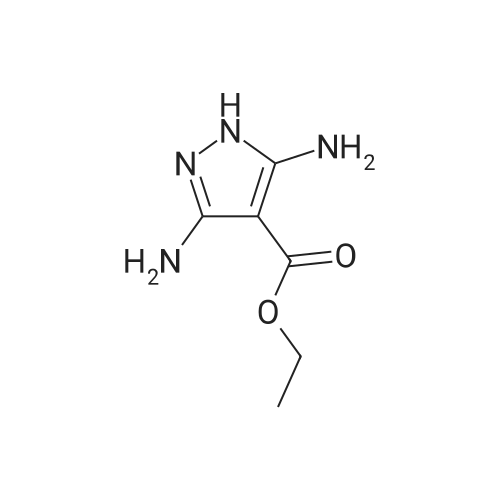
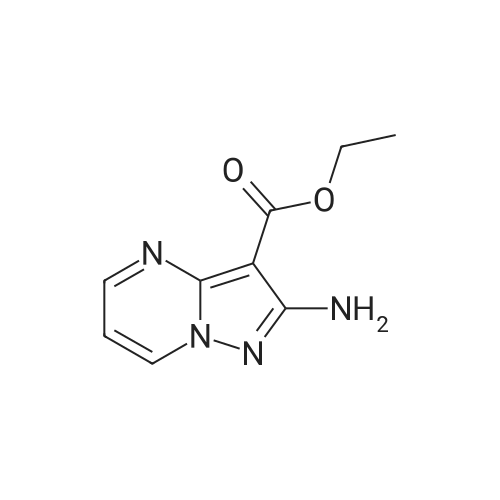
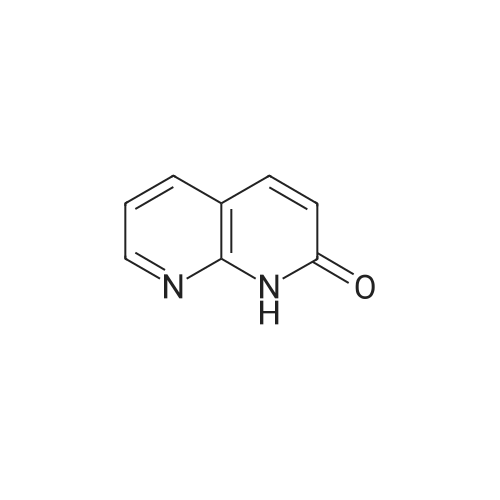
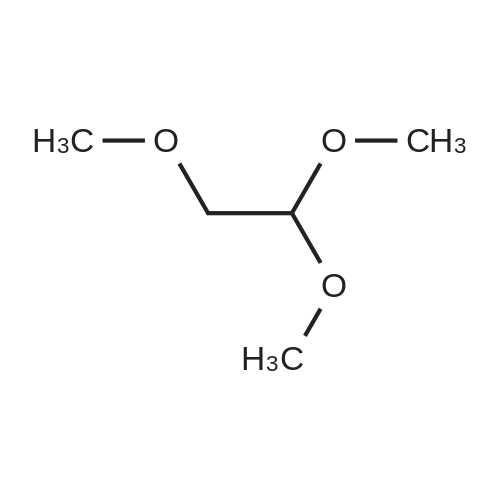
To a solution of ethyl 3,5-diamino-lH-pyrazole-4-carboxylate (5.00 g, 29.38 mmol) in DMF (80 mL) were added 1 , 1 ,3,3-tetramethoxypropane (14.50 mL, 88.15 mmol) and AcOH (0.34 mL, 5.88 mmol). The reaction mixture was stirred at 100 C for 14 h, and then concentrated in vacuo. The residue was partitioned between DCM (50 mL) and water (50 mL). The organic phase was separated and the aqueous phase was extracted with DCM (100 mL x 3). The combined organic phases were washed with brine (100 mL), dried over anhydrous Na2S04, filtered and concentrated in vacuo. The residue was purified by a silica gel column chromatography (a solution of NH3in MeOH (7 M)/DCM (v/v) =1/100) to give the title compound as a pale yellow solid (3.52 g, 58.1%).MS (ESI, pos. ion) m/z: 207.1 [M+H]+; H NMR (400 MHz, CDC1 ): delta (ppm) 8.60 (dd, J= 4.40 Hz, 1.76 Hz, 1H), 8.46 (dd, J = 6.76 Hz, 1.76 Hz, 1H), 6.86 (dd, J = 6.72 Hz, 4.40 Hz, 1H), 4.50 (q, J = 7.08 Hz, 2H), 1.47 (t, J = 7.08 Hz, 3H). |
| 58.1% |
With acetic acid; In N,N-dimethyl-formamide; at 100℃; for 14.0h; |
To a solution of ethyl 3,5-diamino-1 -hydro-pyrazole-4-carboxylate (5.00 g, 29.38 mmol)In N, N-dimethylformamide ((80 mL)1,1,3,3-tetramethoxypropane (14.50 mL, 88.15 mmol)And acetic acid (0.34 mL, 5.88 mmol).After the reaction mixture was stirred at 100 C for 14 hours,Concentrate under reduced pressure. The resulting residue was dispersed in a mixed system of dichloromethane (50 mL) and water (50 mL), the organic phase was separated and the aqueous phase was extracted with dichloromethane (100 mL x 3). The combined organic phases were washed with saturated brine (100 mL x 3), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (NH3 in methanol (7M) / methylene chloride (v / v) = 1/100) to give the title compound as a pale yellow solid (3.52 g, 58.1%). |
| 35.3% |
With triethylamine; In N,N-dimethyl-formamide; at 100℃; for 14.0h; |
ethyl 2-aminopyrazolo[l ,5-a]pyrimidine-3-carboxylateA mixture of ethyl 3,5-diamino-l H-pyrazole-4-carboxylate (1.0 g, 5.9 mmol), 1,1,3,3- tetramethoxypropane (2.9 mL, 18 mmol), triethylamine (2 mL, 10 mmol), and DMF (15 mL) was heated at 100 0C for 14 hrs, then a further 2 mL of 1,1,3,3-tetramethoxypropane was added. After adding the additional 1,1,3,3-tetramethoxypropane, a significant by-product was noted and heating was stopped immediately. The reaction was cooled to room temperature and the DMF was removed in vacuo. The residue was partitioned between DCM and water, then the organic layer was concentrated and the residue purified by silica chromatography, eluting with 95:5 DCM: 2M methanolic ammonia solution to afford 420 mg (35%) of ethyl 2-aminopyrazolo[l,5- a]pyrimidine-3-carboxylate. 1H NMR (500 MHz, CDCl3) delta 8.57 (dd, J = 4.3, 1.6, IH), 8.43 (dd, J = 6.7, 1.6, IH), 6.84 (dd, J = 6.7, 4.4, IH), 5.52 (s, 2H), 4.48 (q, J= 7.1, 2H), 1.45 (t, J= 7.1, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +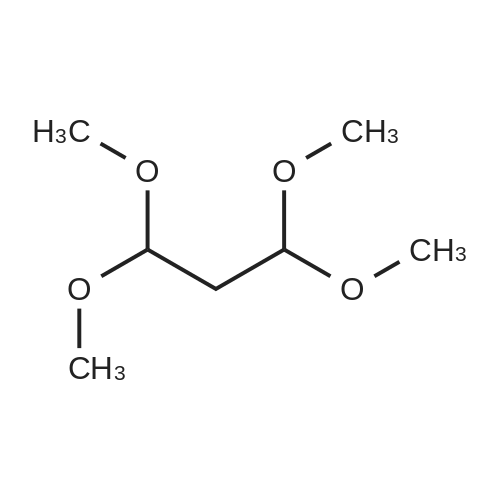

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping