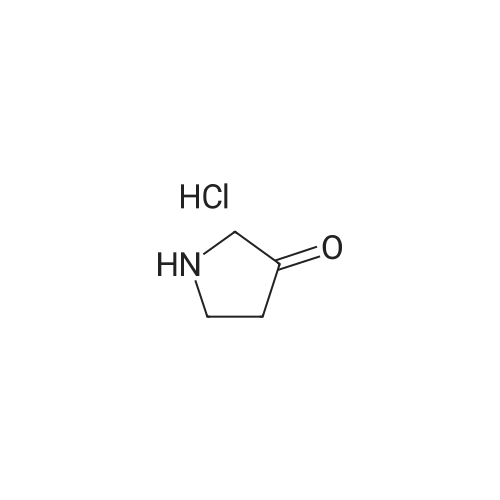
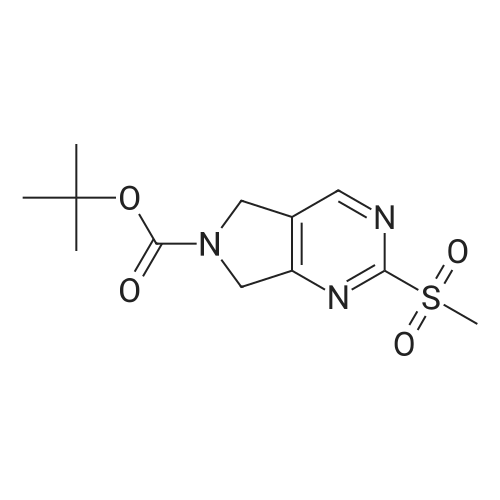
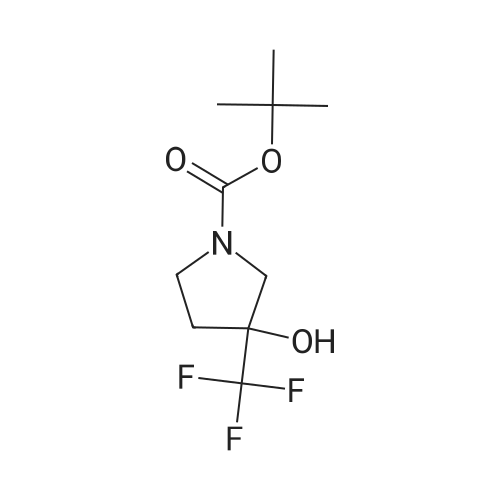
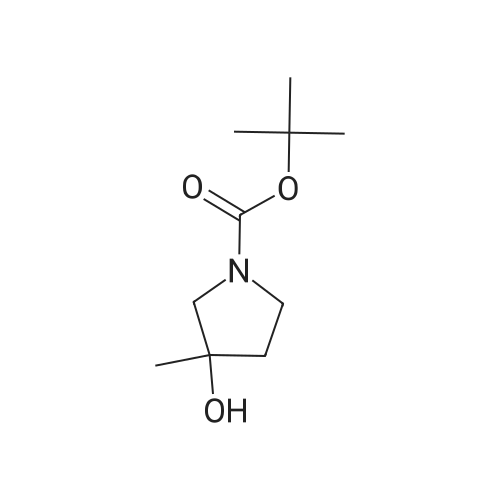
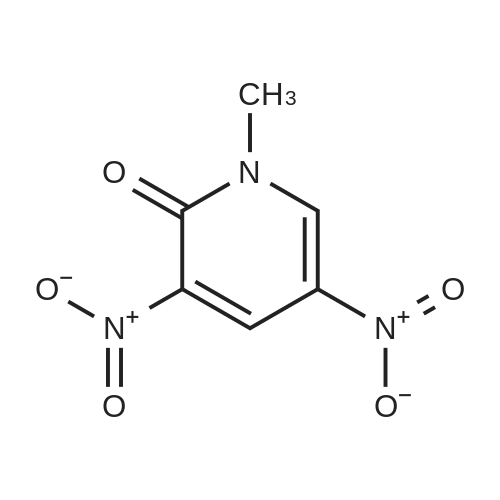
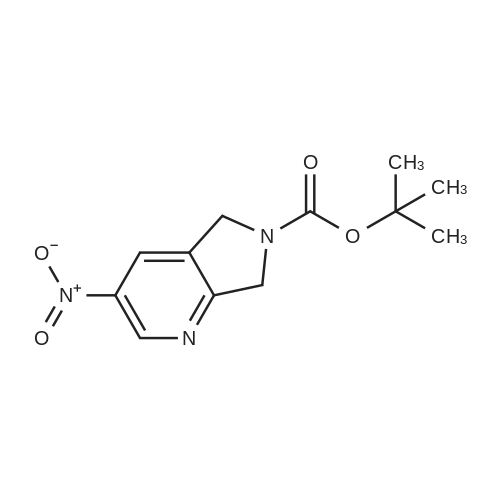
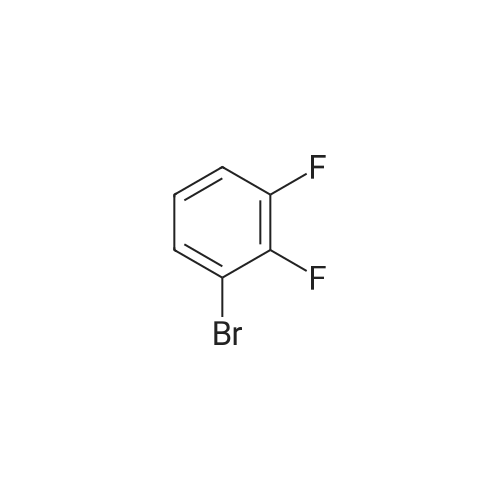
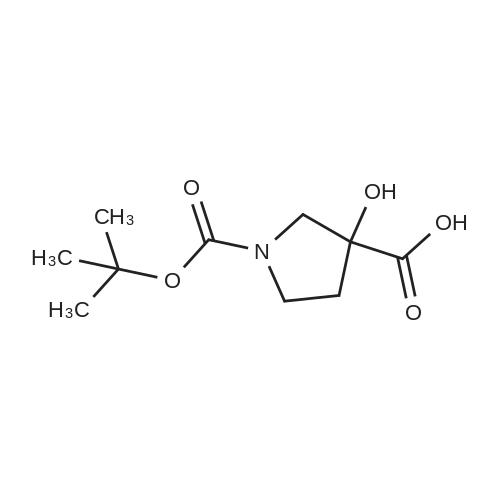
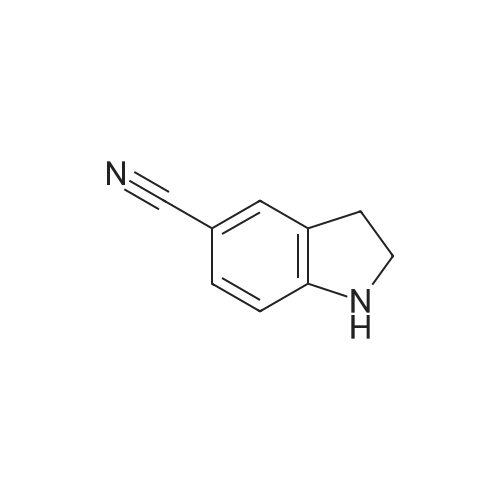
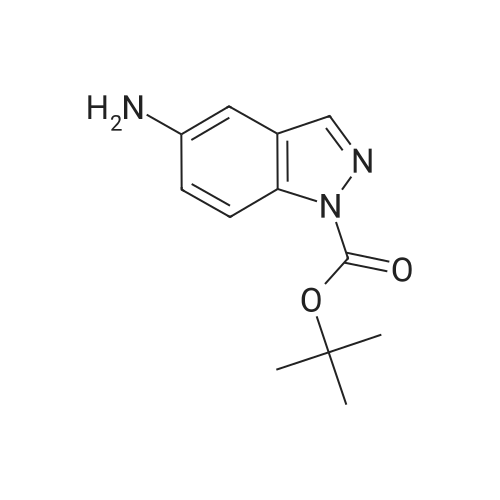
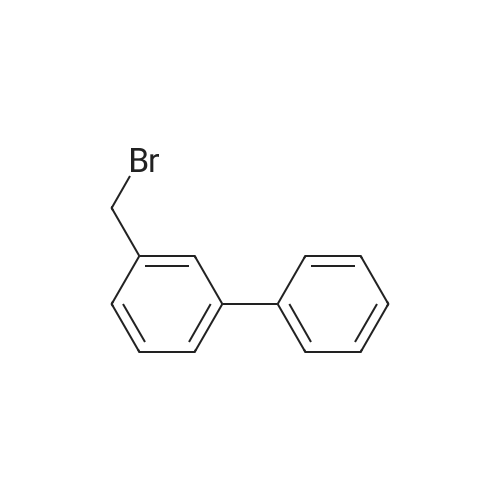
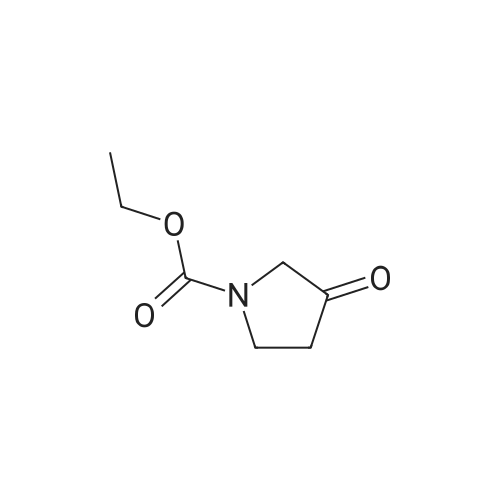
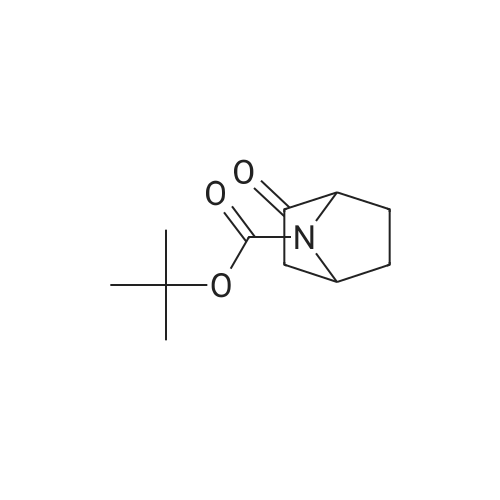
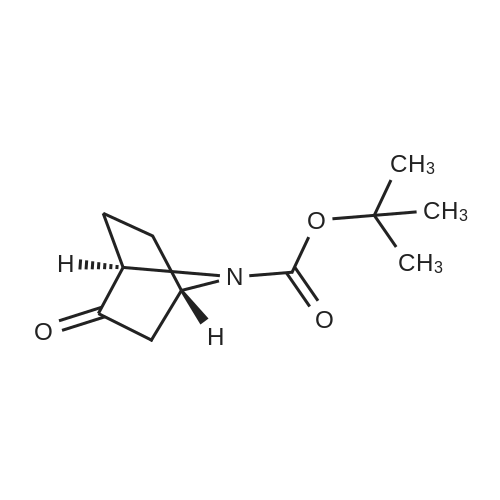
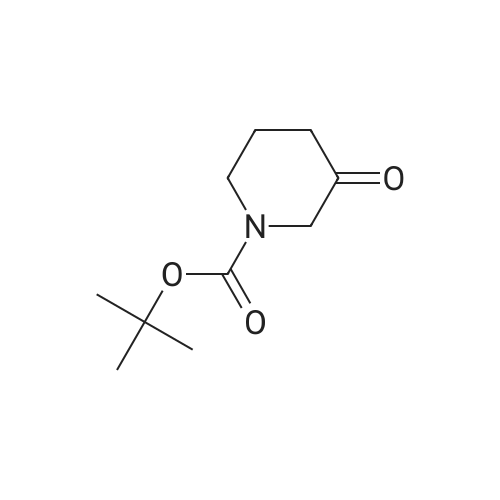
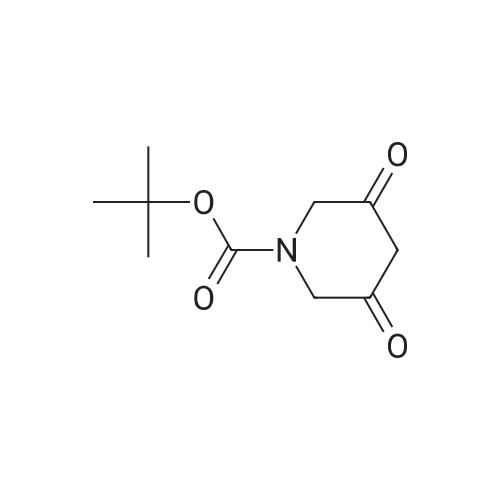
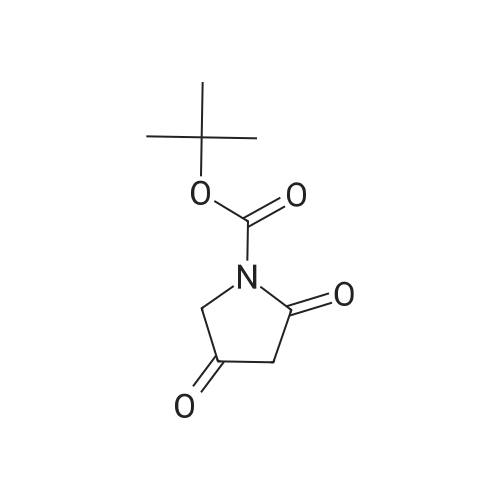
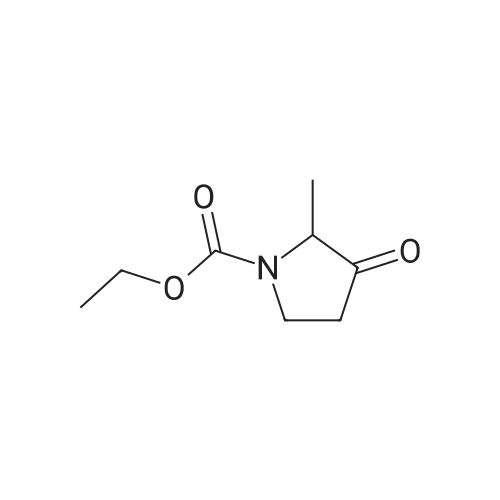
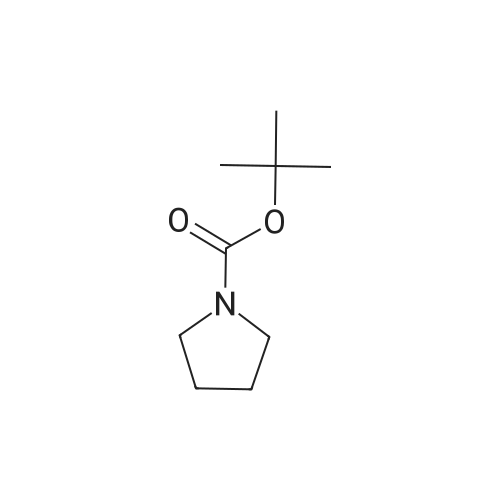
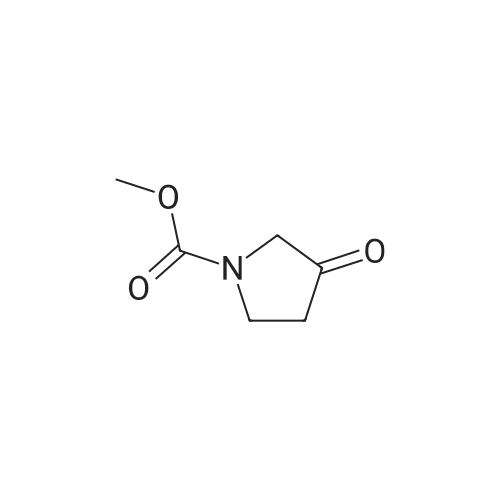
|
With sulfur trioxide trimethylamine complex; triethylamine; In dimethyl sulfoxide; at 20℃; for 18h; |
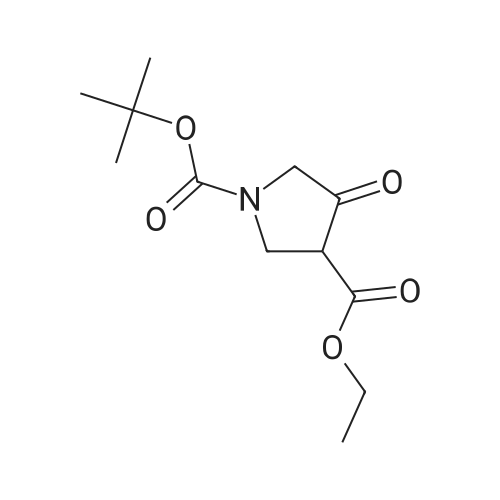
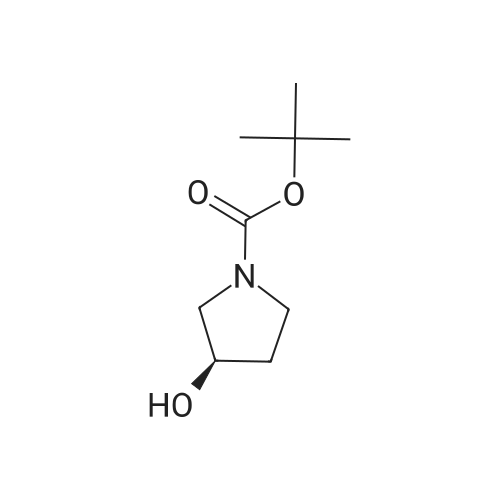
(R)-pyrrolidinol (Tokyo Chemical Industry Co., Ltd., 12.4 g, 100 mmol) was dissolved in 100 ml of a 3 N aqueous solution of sodium hydroxide. A solution (50 ml) of di-tert-butyl dicarbonate (Tokyo Chemical Industry Co., Ltd., 25.0 g, 120 mmol) in tetrahydrofuran was added dropwise thereto at 0C. The pH value of the mixture was determined with a pH test paper and was found to be 11. The mixture was then stirred at room temperature for 2 hr and was then concentrated to remove a major part of tetrahydrofuran. The aqueous layer was extracted three times with ethylacetate. The combined organic layer was dried over sodium sulfate and was concentrated to give a crude product. The crude product and triethylamine (20 ml) were dissolved in anhydrous dimethylsulfoxide (100 ml), and a trituated sulfur trioxide/trimethylamine complex (Aldrich, 28.0 g, 200 mmol) was added little by little thereto at room temperature. The mixture was stirred at room temperature for 18 hr. Water (200 ml) was then added to the reaction solution to stop the reaction. The aqueous layer was extracted three times with ethylacetate. The combined organic layer was dried over sodium sulfate and was concentrated to give a crude product. The crude product was loaded on a silica gel column and developed with chloroform, followed by development with chloroform only to give an intermediate (11.25 g). The intermediate (3.70 g, 20 mmol) and 5-aminoisoquinoline (Aldrich, 2.48 g, 17 mmol) were dissolved in 100 ml of acetic acid. Sodium sulfate (14.2 g, 100 mmol) was added thereto, and the mixture was stirred at room temperature for 30 min. The reaction mixture was cooled to 0C, sodium hydride triacetate (Aldrich, 4.44 g, 20 mmol) was added thereto little by little, and the mixture was stirred at room temperature for 18 hr. The reaction solution was concentrated under the reduced pressure to remove a major part of acetic acid. The reaction mixture was then adjusted to pH = 8 by the addition of a saturated sodium hydrogencarbonate solution and was filtered through Celite, and the filtrate was separated into an organic layer and an aqueous layer. The aqueous layer was extracted three times with ethylacetate. The combined organic layer was dried over sodium sulfate and was concentrated to give a crude product. The crude product in methylene chloride was loaded on a silica gel column and developed with hexane. The development was first carried out with hexane only, subsequently with hexane/chloroform (1 : 1), and finally with chloroform only to collect a fraction having UV absorption with Rf = 0.6 to give the title compound (3.70 g, 12 mmol). 1H-NMR (CDCl3, 400 MHz): 1.46 (s, 9H), 1.75 - 1.94 (m, 1H), 2.02 - 2.10 (m, 1 H), 3.35 - 3.55 (m, 31 H), 3.75 - 3.86 (m, 1 H), 4.17 - 4.24 (m, 1 H), 4.705 - 4.90 (m, 1 H), 6.91 (d, J = 7.6 Hz, 1H), 7.44 (d, J = 8.3 Hz, 1 H), 7.58 (t, J = 7.9 Hz, 1 H), 7.80 - 7.90 (m, 1 H), 8.42 (d, J = 6.4 Hz, 1 H), 9.20 (s, 1 H). Mass spectrometric value (ESI-MS, m/z): 314 (M++1) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping