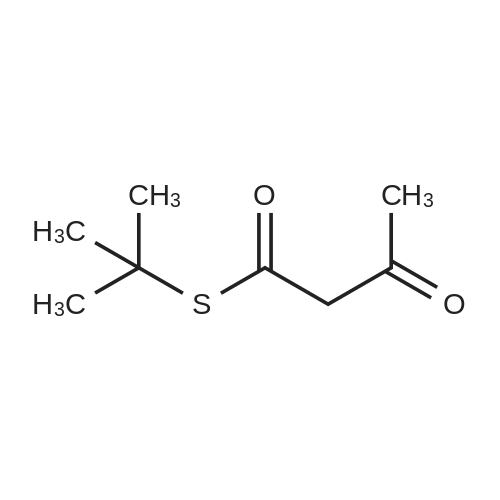
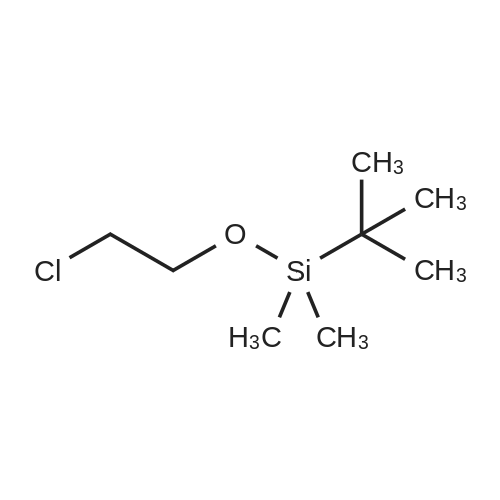
| 100% |
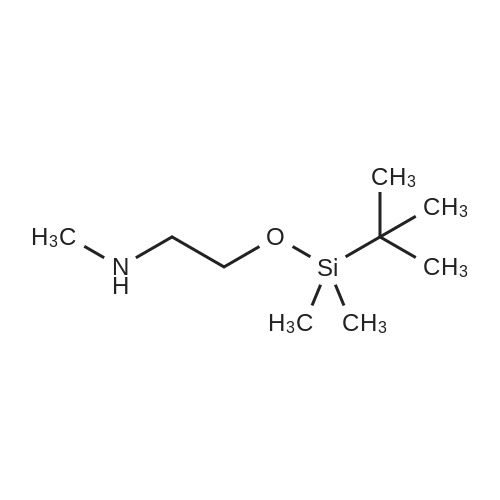
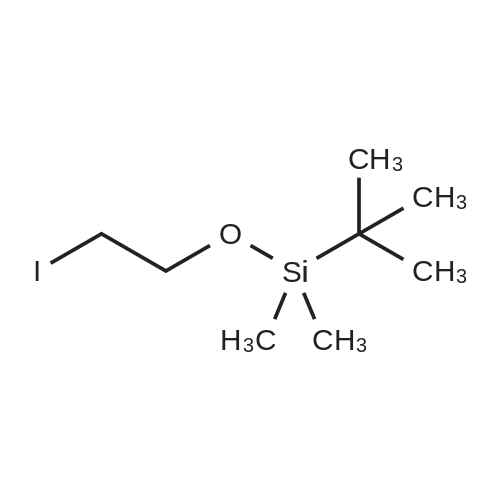
With sodium iodide; In acetone; at 120℃; for 4h;Inert atmosphere; Sealed tube; Microwave irradiation; |
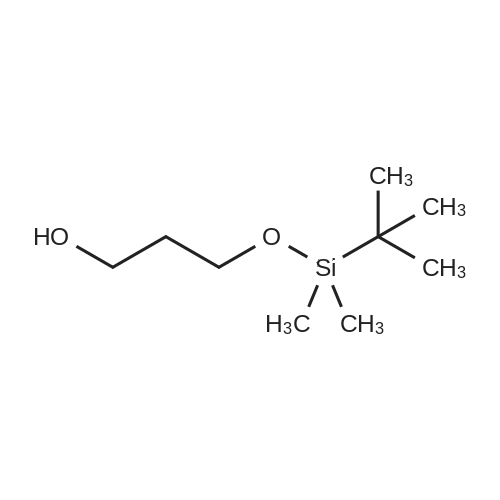
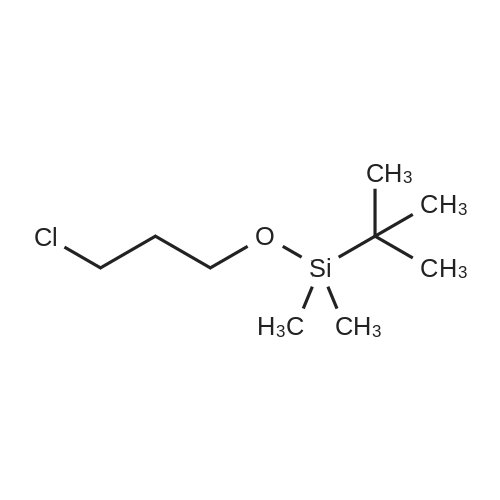
A solution of TBSCl (30.1 g, 200 mmol) in DCM (50 mL) was added dropwise over 15 min to a stirred solution of 2-chloroethanol (16.0 g, 200 mmol), imidazole (20.4 g, 300 mmol) and DMAP (0.244 g, 2.0 mmol) in DCM (200 mL) at 0 °C under an argon atmosphere, and the resulting mixture was warmed to room temperature where it was stirred for 12 h. The mixture was quenched with saturated aqueous NH4Cl (200 mL) and the separated aqueous layer was then washed with DCM (2*100 mL). The combined organic extracts were washed with saturated aqueous NH4Cl (100 mL) and brine (100 mL), then dried over MgSO4 and concentrated to leave 2-chloroethoxy tert-butyl dimethylsilane (37.1 g, 95percent) as a colourless oil. A solution of the silyl ether (3.88g, 20.0mmol) and sodium iodide (8.94g, 60 mmol) in acetone (10mL) was sealed in a microwave vial and heated at 120°C for 4h in a microwave reactor. The vial was cooled to room temperature and the reaction mixture was then diluted with diethyl ether (80mL) and filtered through silica. The filtrate was concentrated to leave 2-iodoethoxy tert-butyl dimethylsilane (5.72g, quant.) as a pale yellow oil, which was used without further purification. A solution of methyl acetoacetate (2.55g, 22.0mmol) in 79 THF (10mL) was added over 10 min to a stirred suspension of sodium hydride (0.88g, 22.0 mmol, 60percent) in THF (50mL) at 0°C under argon. The mixture was stirred for 15 min, and then a solution of n-butyl lithium (11.0 mL, 22.0 mmol, 2.0M in hexane) was added dropwise over 10 min and the mixture was stirred at 0°C for 15 min. A solution of the above, crude iodide (ca. 20 mmol) in THF (10 mL) was added dropwise over 10 min, and the resulting yellow mixture was then warmed to room temperature where it was stirred for 12 h. The mixture was quenched with saturated aqueous NH4Cl (100 mL) and diethyl ether (100mL), and the separated aqueous layer was then washed with diethyl ether (2×100mL). The combined organic extracts were washed with brine (100 mL), then dried over MgSO4 and concentrated to leave a pale yellow gum. Purification by column chromatography (25:1, petrol/Et2O) gave the keto ester (5.05 g, 77percent) as a pale yellow oil (10percent enol form): deltaH (400 MHz, CDCl3) 3.73 (3H, s, CO2CH3), 3.61 (2H, t, J=6.0, CH2O), 3.46 (2H, s, CH2), 2.62 (2H, t, J=7.1, CH2C(O)), 1.80 (2H, tt, J=6.0, 7.1, CH2), 0.87 (9H, s, SiC(CH3)3), 0.03 (6H, s, SiCH3); deltaC (100 MHz, CDCl3) 202.6 (C=O), 167.6 (CO2CH3), 61.8 (CH2O), 52.2 (CO2CH3), 49.0 (CH2), 39.4 (CH2), 26.5 (CH2), 25.9 (SiC(CH3)3), 18.2 (SiC(CH3)3), ?5.4 (2×SiCH3); HRMS m/z C13H26O4SiNa+ (MNa+) requires 297.1498, found 297.1498. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping