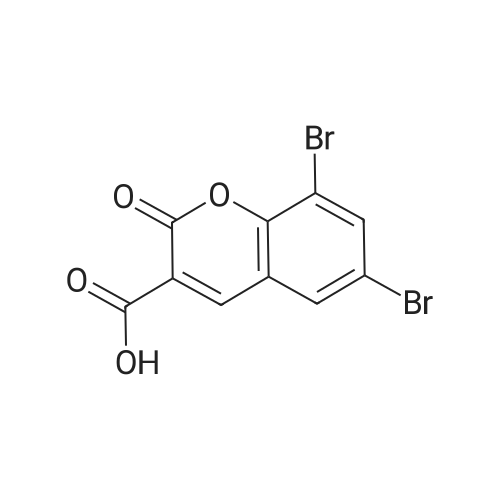
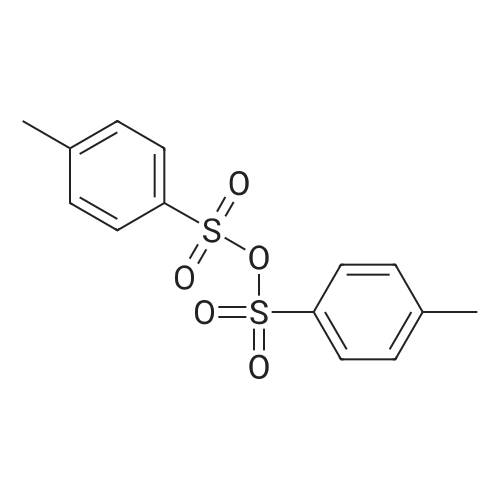
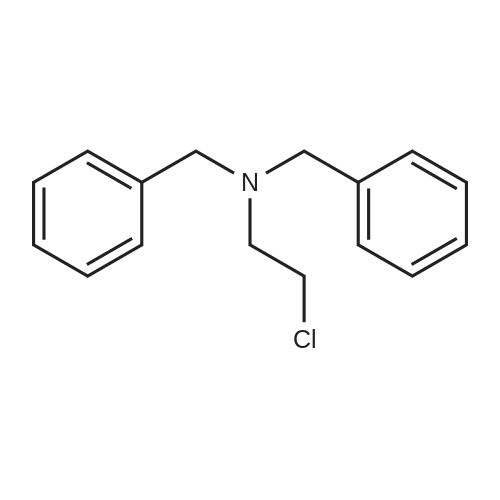
| 83% |
With potassium carbonate; In ethanol; for 16h;Heating / reflux; |
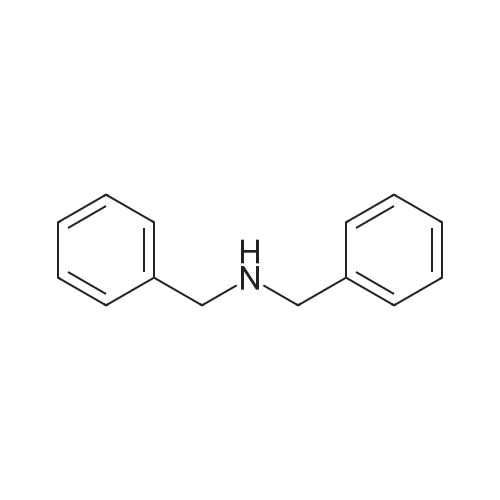
Example 1; Dibenzylaminoethanol:; To a 250 mL round bottom flask equipped with stirbar, reflux condensor, nitrogen inlet, and septum was added potassium carbonate (56.5 g, 0.41 mol), ethanol (80 mL), and ethanolamine (9.9 mL, 0.16 mol). The stirred solution was heated to reflux and benzyl chloride (37.9 mL, 0.33 mol) was added via syringe. The resulting solution was stirred for 16 hours at reflux, allowed to cool, then poured into water (200 mL). The solution was extracted with chloroform (3x300 mL). The combined organic layers were dried with magnesium sulfate, filtered, then evaporated to dryness. Recrystallization from hexanes yielded the product as colorless crystals (32.8 g, 83 %). 1H NMR (300 MHz, CDCl3) delta 7.33 (10 H), 3.60 (4 H), 3.58 (2 H), 2.67 (2H). 13C NMR (75 MHz, CDCl3) delta 138.9, 129.2, 128.6, 127.4, 58.7, 58.4, 55.0. |
| 69% |
With potassium carbonate; In ethanol; for 36h;Reflux; |
Dibenzylamino Ethanol Benzyl chloride (278.5g, 2.2 mol), ethanol amine (60 mL, 1 mol), potassium carbonate (283.1g, 2.05mol) and ethanol (2 L) were mixed together in a 3L 3- neck flask, fitted with an overhead stirrer, a condenser and a glass plug. The apparatus was heated up to reflux for 36 hr, after which the insoluble solid was filtered through a medium frit. The filtrate was recovered and ethanol was removed by rotoary evaporation. The viscous liquid was redissolved in ether, the solid suspension removed by filtration and extracted twice against water. The ether solution was kept and the aqueous layer was extracted twice with dichloromethane (2 x 400 mL). The fraction were recombined, dried over MgSO4, stirred over carbon black for 15 min and filtered through a celite pad. Dichloromethane was removed and the solid was redissolved into a minimal amount of ether (combined volume of 300 mL with the first ether fraction, 300 mL). Hexanes ( 1700 mL) was added and the solution was heated up gently till complete dissolution of the product. The solution was then cooled down gently, placed in the fridge (+ 40C) overnight and white crystals were obtained. The recrystallization was done a second time. 166.63g, 69% yield. 1H NMR (d6-DMSO) delta 7.39-7.24 (10H), 4.42 (IH), 3.60 (4H), 3.52 (2H), 2.52 (2H). |
| 69% |
With potassium carbonate; In ethanol; for 36h;Reflux; |
Benzyl chloride (278.5 g, 2.2 mol), ethanol amine (60 mE, 1 mol), potassium carbonate (283.1 g, 2.05 mol) and ethanol (2 E) were mixed together in a 3 E 3-neck flask, fitted with an overhead stirrer, a condenser and a glass plug. The apparatus was heated up to reflux for 36 hr, after which the insoluble solid was filtered through a medium frit. The filtrate was recovered and ethanol was removed by rotary evaporation. The viscous liquid was redissolved in ether, the solid suspension removed by filtration and extracted twice against water. The ether solution was kept and the aqueous layer was extracted twice with dichioromethane (2x400 mE). The fraction were recombined, dried over MgSO4, stirred over carbon black for 15 mm and filtered through a celite pad. Dichloromethane was removed and the solid was redissolved into a minimal amount of ether (combined volume of 300 mE with the first ether fraction, 300 mE). Rexanes (1700 mE) was added and the solution was heated up gently till complete dissolution ofthe product. The solution was then cooled down gently, placed in the fridge (+4 C.) overnight and white crystals were obtained. The recrystallization was done a secondtime. 166.63 g, 69% yield. ?RNMR(d5-DMSO)oe 7.39- 7.24 (bR), 4.42 (1R), 3.60 (4R), 3.52 (2R), 2.52 (2R). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping