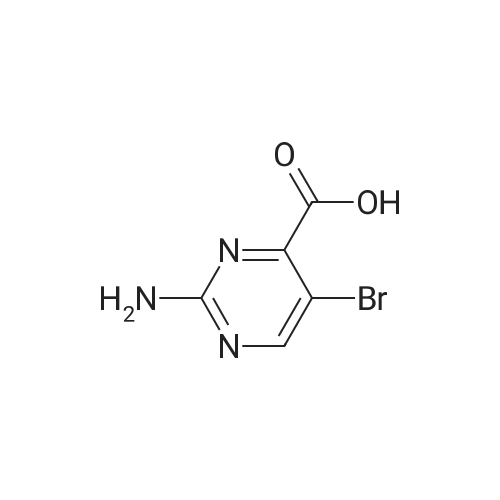
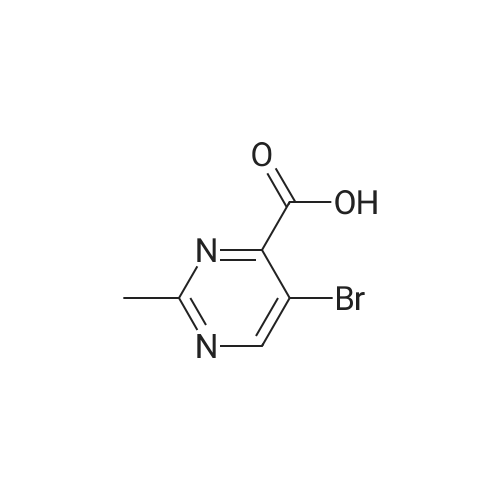
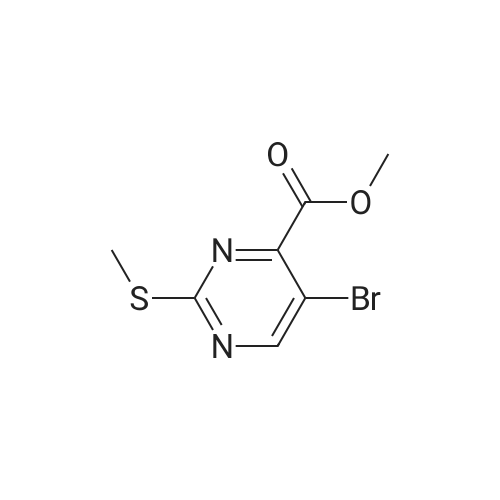
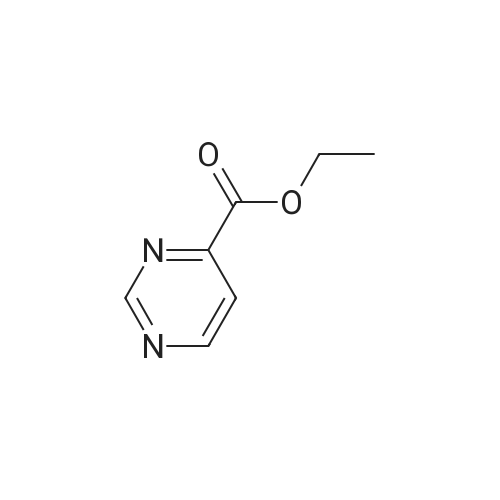
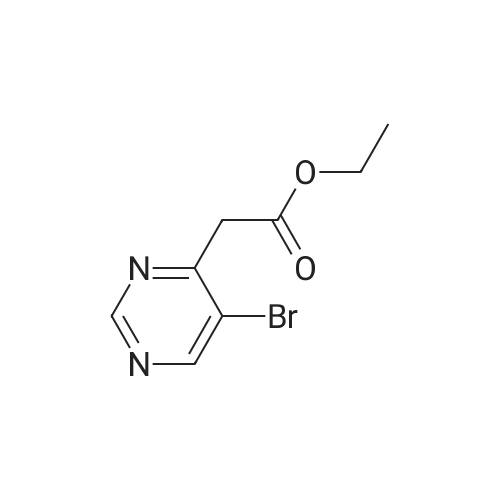
| 8.5% |
With sodium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; In N,N-dimethyl-formamide; at 120℃; for 18.0h; |
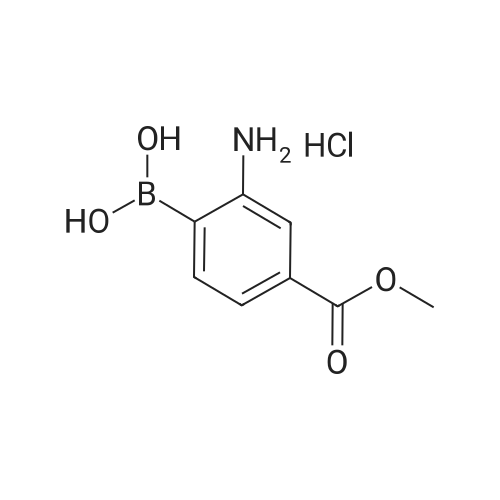
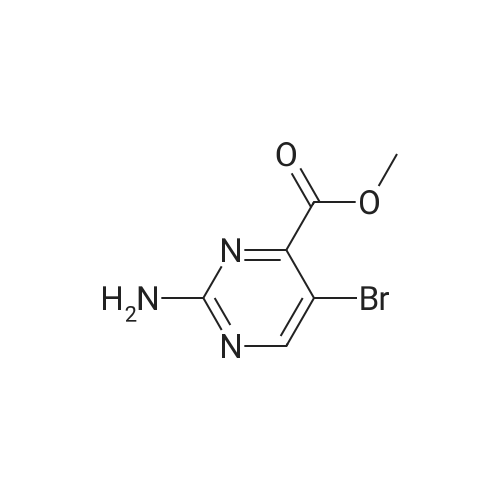
Sodium acetate (4.0 eq, 1.92 g, 23.41 mmol) and 1,1'- bis(diphenylphosphino)ferrocene palladium (II) chloride (complexed with dichloromethane) (0.05 eq, 214 mg, 0.29 mmol) were added to a mixture of methyl-5- bromopyrimidine-4-carboxylate (1.0 eq, 1.27 g, 5.85 mmol), and 2-amino-4- (methoxycarbonyl)phenylboronic acid hydrochloride (1.0 eq, 1.35 g, 5.85 mmol) in anydrous DMF (10 ml). The Mixture was stirred under nitrogen atmosphere at 1200C for 18 hours. Water and brine were added and the resulting solid impurities filtered off. The material was extracted with CH2Cl2 (4x) and the combined extracts dried over Na2SO4. After evaporation of CH2Cl2, the remaining DMF was evaporated by heating the residue in vacuo. The resulting solid was triturated in CH2Cl2, filtered and <n="76"/>dried to provide methyl 5-oxo-5,6-dihydropyrimido[4,5-c]quinoline-8-carboxylate as a beige solid (127 mg, 8.5percent yield). LCMS (ES): >80percent pure, m/z 256 [M+l]+; 1H NMR (DMSO-^6, 400 MHz) delta 3.79 (s, 3H), 7.81 (d, / = 8.0, IH), 8.68 (d, / = 8.8, IH), 9.49 (s, IH), 10.19 (s, IH), 12.37 (s, IH) ppm. |
| 8.5% |
With sodium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In N,N-dimethyl-formamide; at 120℃; for 18.0h; |
Process 5 Sodium acetate (4.0 eq, 1.92 g, 23.41 mmol) and 1,1'-bis(diphenylphosphino)ferrocene palladium (II) chloride (complexed with dichloromethane) (0.05 eq, 214 mg, 0.29 mmol) were added to a mixture of methyl-5-bromopyrimidine-4-carboxylate (1.0 eq, 1.27 g, 5.85 mmol), and <strong>[380430-55-7]2-amino-4-(methoxycarbonyl)phenylboronic acid hydrochloride</strong> (1.0 eq, 1.35 g, 5.85 mmol) in anhydrous DMF (10 ml). The mixture was stirred under nitrogen atmosphere at 120° C. for 18 hours. Water and brine were added and the resulting solid impurities filtered off. The material was extracted with CH2Cl2 (4.x.) and the combined extracts dried over Na2SO4. After evaporation of CH2Cl2, the remaining DMF was evaporated by heating the residue in vacuo. The resulting solid was triturated in CH2Cl2, filtered and dried to provide methyl 5-oxo-5,6-dihydropyrimido[4,5-c]quinoline-8-carboxylate as a beige solid (127 mg, 8.5percent yield). LCMS (ES): >80percent pure, m/z 256 [M+1]+; 1H NMR (DMSO-d6, 400 MHz) delta 3.79 (s, 3H), 7.81 (d, J=8.0, 1H), 8.68 (d, J=8.8, 1H), 9.49 (s, 1H), 10.19 (s, 1H), 12.37 (s, 1H) ppm. |
|
With sodium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; In N,N-dimethyl-formamide; at 120℃; for 18.0h;Inert atmosphere; |
Sodium acetate (4.0 eq, 1.92 g, 23.41 mmol) and 1,1'-bis(diphenylphosphino)ferrocene palladium (II) chloride (complexed with dichloromethane) (0.05 eq, 214 mg, 0.29 mmol) were added to a mixture of methyl-5-bromopyrimidine-4-carboxylate (1.0 eq, 1.27 g, 5.85 mmol), and <strong>[380430-55-7]2-amino-4-(methoxycarbonyl)phenylboronic acid hydrochloride</strong> (1.0 eq, 1.35 g, 5.85 mmol) in anhydrous DMF (10 ml). The Mixture was stirred under nitrogen atmosphere at 120° C. for 18 hours. Water and brine were added and the resulting solid impurities filtered off. The material was extracted with CH2Cl2 (4.x.) and the combined extracts dried over Na2SO4. After evaporation of CH2Cl2, the remaining DMF was evaporated by heating the residue in vacuo. The resulting solid was triturated in CH2Cl2, filtered and dried to provide methyl 5-oxo-5,6-dihydropyrimido[4,5-c]quinoline-8-carboxylate as a beige solid (127 mg, 8.5percent yield). LCMS (ES): >80percent pure, m/z 256 [M+1]+; 1H NMR (DMSO-d6, 400 MHz) delta 3.79 (s, 3H), 7.81 (d, J=8.0, 1H), 8.68 (d, J=8.8, 1H), 9.49 (s, 1H), 10.19 (s, 1H), 12.37 (s, 1H) ppm. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping