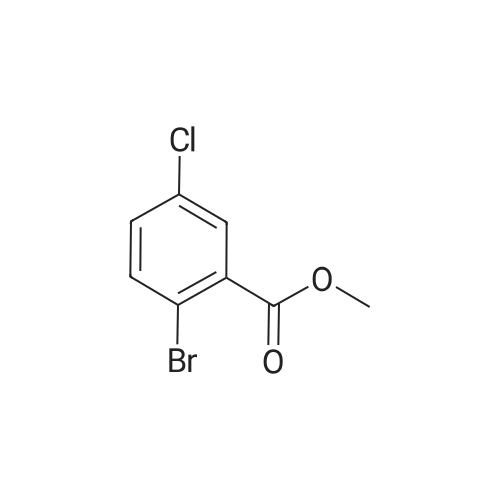
|
With sodium carbonate;tetrakis(triphenylphosphine) palladium(0); In ethanol; water; toluene; at 90℃; for 24h; |
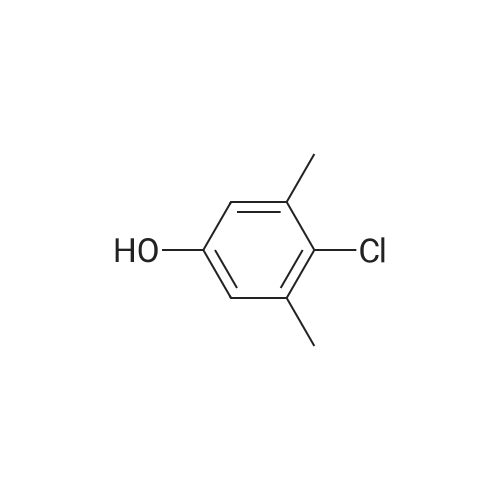
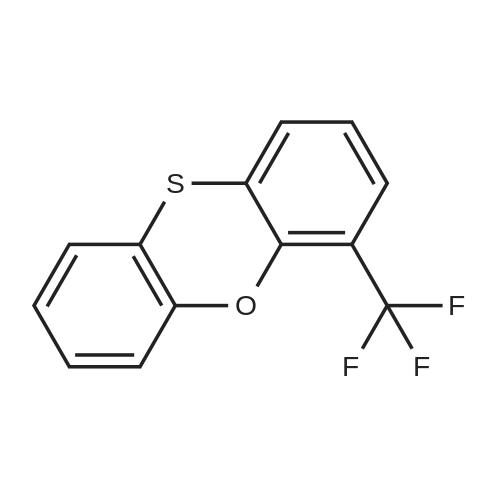
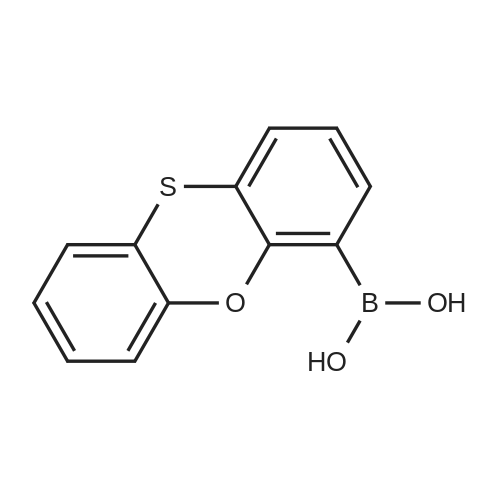
N,N-Diethyl-4-(4-phenoxathiinyl-4-piperidinylidenemethyl)- benzamide To a mixture of INTERMEDIATE 5 (0.202 g, 0.448 mmol), 4-PHENOXATHIINYL boronic acid (0.164 g, 0.672 MMOL), toluene (3.6 mL), ethanol (0.84 mL), and 2.0 M NA2CO3 (0.56 mL) in a N2 purged vial was added palladium tetrakistriphenylphosphine (0. 0518 g, 0.045 mmol). The resulting mixture was heated at 90 C for 24 h. The reaction was then concentrated in vacuo and the residue was diluted with water (4 mL) and CH2CI2 (4 mL). The layers were separated, and the aqueous phase was extracted with CH2CI2. The combined organic phases were dried over NA2SO4, filtered, and concentrated in vacuo. The crude product was purified by silica gel column chromatography eluting with 5: 1 CH2CI2 : EtOAc. The purified product was dissolved in CH2CI2 (10 mL) and trifluoroacetic acid (1 mL) was added. After 1.5 h, the reaction was concentrated in vacuo. The residue was LYOPHILIZED from CH3CN/H20 to give COMPOUND 3 as its trifluoroacetic acid salt (0.250 g, 95%) as a slightly yellow solid. Purity (HPLC): > 99% ; H NMR (400MHZ, CD30D) 8 1.06 (br t, J = 6.9 Hz, 3H), 1.22 (br t, J = 7.2 Hz, 3H), 2.44-2. 58 (m, 2H), 2.63-2. 73 (m, 1 H), 2.74-2. 83 (m, L H), 3.18-3. 28 (m, 5H), 3.31-3. 40 (m, 1 H), 3.52 (br q, J = 7.4 Hz, 2H), 6.75 (dd, J = 1.3 Hz, 7.9 Hz, IH), 6.98-7. 13 (m, 5H), 7.14-7. 17 (M, 1H), 7. 35 (s, 4H). |
|
With sodium carbonate;tetrakis(triphenylphosphine) palladium(0); In ethanol; toluene; at 90℃; for 24h; |
To a mixture of INTERMEDIATE 5 (0.202 g, 0.448 MMOL), 4-phenoxathiinyl boronic acid (0.164 g, 0.672 MMOL), toluene (3.6 mL), ethanol (0.84 mL), and 2.0 M NA2CO3 (0.56 mL) in a N2 purged vial was added palladium tetrakistriphenylphosphine (0. 0518 g, 0.045 MMOL). The resulting mixture was heated at 90 C for 24 h. The reaction was then CONCENTRATED IN VACUO and the residue was diluted with water (4 mL) and CH2CI2 (4 mL). The layers were separated, and the aqueous phase was extracted with CH2CI2. The combined organic phases were dried over NA2SO4, filtered, and CONCENTRATED IN VACUO. The crude product was purified by silica gel column chromatography eluting with 5: 1 CH2CI2 : EtOAc. The purified product was dissolved in CH2CI2 (10 mL) and trifluoroacetic acid (I mL) was added. After 1. 5 h, the reaction was CONCENTRATED IN VACUO. The residue was LYOPHILIZED from CH3CN/H20 to give COMPOUND 3 as its trifluoroacetic acid salt (0.250 g, 95%) as a slightly yellow solid. Purity (HPLC) : > 99% ; H NMR (400MHZ, CD30D) 8 1.06 (br t, J = 6.9 Hz, 3H), 1.22 (br t, J = 7.2 Hz, 3H), 2.44-2. 58 (m, 2H), 2.63-2. 73 (M, 1H), 2.74-2. 83 (m, 1 H), 3.18-3. 28 (m, 5H), 3. 31-3. 40 (m, 1H), 3.52 (br q, J = 7.4 Hz, 2H), 6.75 (dd, J = 1. 3 Hz, 7.9 Hz, 1H), 6.98-7. 13 (m, 5H), 7.14-7. 17 (m, 1 H), 7. 35 (s, 4H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping