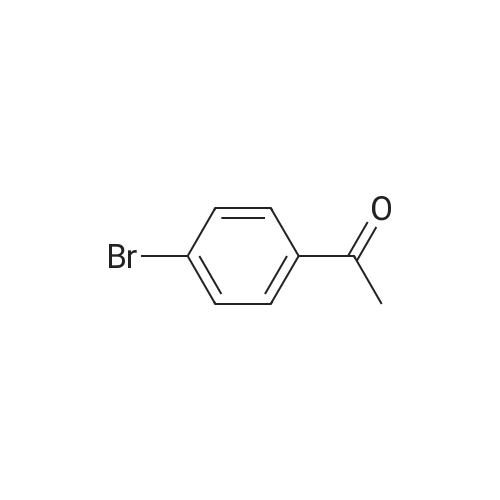
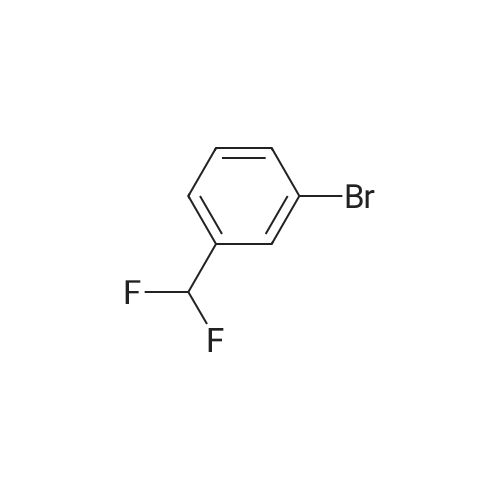
| 59% |
at 85℃; for 15 h; |
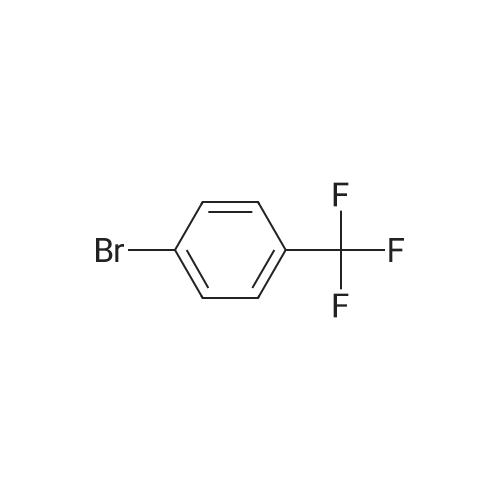
Deoxo-Fluor (registered trademark) (22.2 g) was added to 1-(4-bromophenyl)ethanone (20.0 g) and the mixturewas stirred at 85°C for 15 hours. Under icecooling, ice water and an aqueous solution of potassium carbonate wereadded to the reaction solution, followed by extraction with chloroform. The solvent was evaporated under reducedpressure and the obtained residue was purified by silica gel column chromatography (hexane) to give the title compound(13.0 g, yield 59percent) as a yellow oil.1H NMR (600 MHz, CDCl3) δ ppm 1.91 (t, J=18.2 Hz, 3H), 7.50 (d, J=8.3 Hz, 2H), 7.86 (d, J=8.3 Hz, 2H). |
| 25% |
With (bis-(2-methoxyethyl)amino)sulfur trufluoride In dichloromethane; toluene at 20 - 85℃; for 20 h; Ionic liquid; Sealed tube |
Step A: Preparation of l-bromo-4-(l,l-difluoroethyl)benzene. [00211] To a vial equipped with a magnetic stir bar were added a solution of l-(4- bromophenyl)ethanone (295 mg, 1.48 mmol) in anhydrous DCM (3.0 mL) followed by a 50percent solution of Deoxofluor? in toluene (1.6 mL, 4.45 mmol) at room temperature under N2 and the vial was sealed. The reaction mixture was stirred for approximately 15 h at room temperature, but little conversion had taken place. The mixture was concentrated, treated with additional Deoxofluor? solution (0.66 mL, 1.79 mmol),and warmed to and stirred at 85 °C under N2 for 5 h. The reaction mixture was cooled to 0 °C and carefully quenched by adding sat'd aq NaHC03 dropwise until gas evolution ceased. The bi-phasic mixture was extracted with DCM (2 x 5 mL), and the combined extracts were dried over Na2S04, filtered, and concentrated. The residue was purified by column chromatography (Si02, 0-^50 EtOAc in hexanes) to afford the title compound (83 mg, 25percent)as a clear liquid: 1H NMR (400 MHz, CDC13) δ 7.58 - 7.53 (m, 2H), 7.41 - 7.35 (m, 2H), 1.90 (t, J = 18.1 Hz, 3H); 19F NMR (376 MHz, CDC13) δ -87.86; IR (Thin Film) 1599, 1294, 1089 cm"1; EIMS mlz 220/221. |
| 17.26 g |
With (bis-(2-methoxyethyl)amino)sulfur trufluoride In chloroform at 50℃; for 35 h; Inert atmosphere |
Under argon atmosphere, into a 500-ml reaction vessel made of tetrafluoroethylene-perfluoroalkyl vinyl ether copolymer (PFA) and equipped with a stirring apparatus were placed 25 g (126 mmol) of 4-bromoacetophenone, 111 g (500 mmol) of bis(2-methoxyethyl)aminosulfur trifluoride, and 250 ml of anhydrous chloroform, so that a homogeneous solution was prepared. And then, the solution was reacted at an internal temperature of about 50°C for 35 hours. Subsequently, the reaction solution was cooled to room temperature, and then the reaction solution was added to 1000 ml of a saturated aqueous solution of sodium hydrogen carbonate, which was cooled in ice. Subsequently, the mixture was subjected to extraction with 500 ml of chloroform. The solvent was distilled off under a reduced pressure, and then the reaction mixture was purified by silica gel column chromatography (hexane: 100 vol percent), to provide 17.26 g of Compound (4-1) in the form of a colorless liquid. [0134] The properties of Compound (4-1) were as follows. 1H-NMR (400MHz, CDCl3, 8 (ppm)); 1.90 (3H, t, J=18.1Hz), 7.54 (2H, d, J=2.3Hz), 7.57 (2H, d, J=2.4Hz) CI-MS; 222 (M+2) |
| 17.26 g |
With (bis-(2-methoxyethyl)amino)sulfur trufluoride In chloroform at 50℃; for 35 h; Inert atmosphere |
Under argon atmosphere, into a 500-ml reaction vessel 30 made of tetrafluoroethylene-perfluoroalkyl vinyl ether copolymer (PFA) and equipped with a stirring apparatus were placed 25 g (126 mmol) of 4-bromoacetophenone, 111 g (500 mmol) of bis(2-methoxyethyl)aminosulthr trifluoride, and 250 ml of anhydrous chloroform, so that a homogeneous solution was prepared. And then, the solution was reacted at an internal temperature of about 50° C. for 35 hours. Subsequently, the reaction solution was cooled to room temperature, and then the reaction solution was added to 1000 ml of a saturated aqueous solution of sodium hydrogen carbonate, which was cooled in ice. Subsequently, the mixture was subjected to extraction with 500 ml of chloroform. The solvent was distilled off under a reduced pressure, and then the reaction mixture was purified by silica gel column chromatography (hexane: 100 vol percent), to provide 17.26 g of Compound (4-1) in the form of a colorless liquid.The properties of Compound (4-1) were as follows. ‘H-NMR (400 MHz, CDC13, ? (ppm)); 1.90 (3H, t, J=18. 1 Hz), 7.54 (2H, d, J=2.3 Hz), 7.57 (2H, d, J=2.4 Hz)CI-MS; 222 (M+2)4045 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping