
Vinorelbine tartrate NEW
| Price | Get Latest Price |
| Package | 25kg |
| Min. Order: | 1kg |
| Supply Ability: | 2000ton |
| Update Time: | 2024-04-12 |
Product Details
| Product Name: Vinorelbine tartrate | CAS No.: 125317-39-7 |
| EC-No.: 639-264-2 | Min. Order: 1kg |
| Purity: 99% | Supply Ability: 2000ton |
| Release date: 2024/04/12 | |
| Appearance: White Powder |
Vinorelbine tartrate is a derivative of vincristine and acts similar to vincristine. It was first sold in France in 1989. The main function is to bind to tubulin, which causes the formation of microtubules in cells during mitosis. Vinorelbine is a cycle-specific drug, which is often used in the treatment of non-small cell lung cancer (NSCLC), breast cancer, ovarian cancer, head and neck phosphorous cancer, and leukemia, and also has a strong inhibitory effect on small cell lung cancer, colon cancer, brain tumor, malignant melanoma, etc.
Pharmacological action
Vinorelbine tartrate is the tartrate form of vinorelbine, which is a semi-synthetic vinblastine compound and an M-phase specific drug with a similar mechanism of action to vincristine. By selectively blocking tubulin polymerization to form microtubules in mitotic cells, tubulin depolymerization can be induced, which interferes with spindle microtubules and stops cell division in the middle stage, but has little effect on tubulin synthesis of axons in nerve cells. Because NVB has a poor affinity for axonal microtubules and has an effect on axons only at high concentrations, it is less neurotoxic than other vinblastine drugs and has a greater therapeutic index. The human pharmacokinetic study of vinorelbine showed that after intravenous administration, it was presented as a three-compartment model with large distribution volume, PPB up to 50%-80%, high plasma clearance rate, terminal clearance phase T1/2 of 40h, faster tissue distribution, and its tissue concentration was significantly higher than that of vincrixin (VCR) and vindesine (VDS). The plasma clearance rate was 0.8L/(kg·h), which was mainly secreted by biliary tract and excreted with feces, and urine excretion accounted for 10% ~ 15%. The efficacy is higher and the nervous system side effects are less.
Adverse reactions and side effects
① The hematological toxicity of vinorelbine was dose-limited, mainly leukopenia, mostly recovered within 7 days, and had certain effects on red blood cells, with thrombocytopenia and anemia occurring in less than 2%.
② Neurotoxicity was mainly manifested as weakened tendon reflex (about 25%), occasional paresthesia, a few patients may have gastrointestinal autonomic paralysis caused by constipation (17% to 41%), paralytic intestinal obstruction is rare, 2% to 6% of patients have numbness of the finger (toe), but the incidence is much lower than VCR and VDS.
③ The incidence of gastrointestinal reactions was less than 10%. Occasionally, nausea, vomiting, dyspnea, and bronchospasm occur within minutes or hours of medication. The incidence of hair loss is less than 10%.
(4) Extravasation of vinorelbine into surrounding tissues can cause burning pain, injection site phlebitis, local tissue necrosis, ulcers, cellulitis, etc.
(5) Myelosuppression was dose limiting toxicity. Temporary myelosuppression can occur 3 to 5 days after medication, and it usually recovers naturally within 10 days. Attention should be paid to preventing infection. Neurotoxicity was observed.
Storage Condition | Keep in a cool and dry place |
Transportation | By Sea or by Air(DHL/UPS/TNT/FEDEX/EMS) |
Delivery Time | 7-28 days |
Payment | T/T, Western Union or Bitcoin |





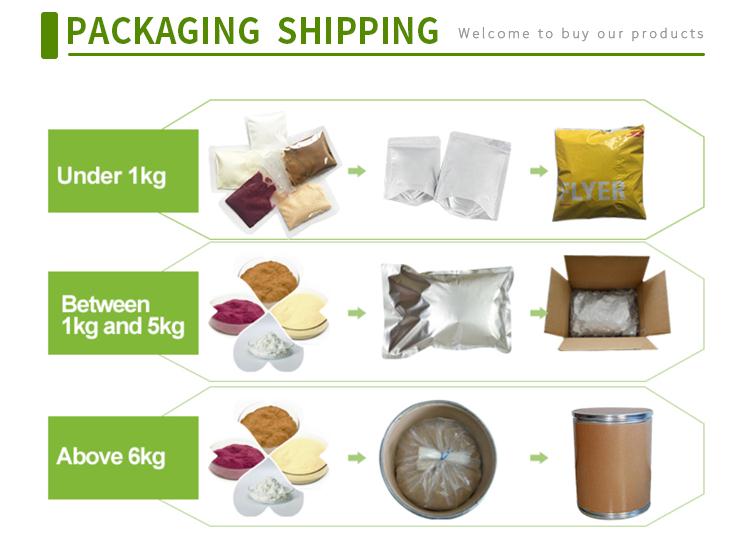




Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $100.00/25KG |
VIP6Y
|
Hebei Weibang Biotechnology Co., Ltd
|
2024-10-31 | |
| $1.00/1G |
VIP1Y
|
S&Y Biochem Co.,Ltd
|
2024-09-23 | |
| $10.70/1kg |
VIP4Y
|
Hebei Chuanghai Biotechnology Co,.LTD
|
2024-08-21 | |
| $15.00/1kg |
Ouhuang Engineering Materials (Hubei) Co., Ltd
|
2024-04-28 | ||
| $50.00/1kg |
Anhui Ruihan Technology Co., Ltd
|
2023-08-23 | ||
| $0.00/25KG |
VIP5Y
|
Hebei Mojin Biotechnology Co., Ltd
|
2023-07-04 | |
| $1.00/1KG |
Weijer International Trade (Hebei) Co., Ltd
|
2023-03-09 | ||
| $0.00/1kg |
VIP3Y
|
Changzhou Rokechem Technology Co., Ltd.
|
2022-11-30 | |
| $1.00/1mg |
ANHUI SHENGZHIKAI BIOTECHNOLOGY CO.,LTD
|
2022-07-07 | ||
| $50.00/1KG |
XINGTAI XINGJIU NEW MATERIAL TECHNOLOGY CO., LTD
|
2022-01-22 |
- Since: 2020-08-05
- Address: 804, Unit 2, Building 4, i City, No. 11, South Tangyan Road, High-tech Zone, Xi 'an, Shaanxi











 China
China