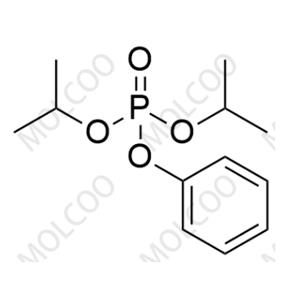
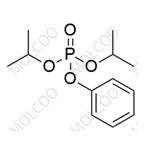
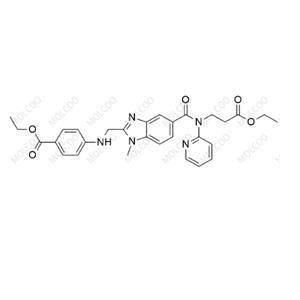
Sofosbuvir Impurity 93 NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 100000000000 |
| Update Time: | 2025-01-15 |
Product Details
| Product Name: Sofosbuvir Impurity 93 | CAS No.: 51496-03-8 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 100000000000 | Release date: 2025/01/15 |
Sofosbuvir Impurity Reference Standards
Sofosbuvir, as a highly effective antiviral drug, plays a crucial role in drug research and development, production, and quality control. The Sofosbuvir impurity reference standards we provide have been carefully prepared and rigorously tested to ensure their accuracy and reliability.
Product Features:
Comprehensive Range: Our product covers a wide range of Sofosbuvir impurities, including Impurity 1 to Impurity 41, to meet your needs at different research stages.
High Purity: All impurity reference standards are purified using advanced techniques such as High Performance Liquid Chromatography (HPLC), with purities exceeding 95%.
Structural Confirmation: Nuclear Magnetic Resonance (NMR) and Mass Spectrometry (MS) spectra are provided to ensure the accurate structure of the impurity reference standards.
Quality Assurance: Each batch of impurity reference standards is accompanied by a detailed Certificate of Analysis (COA) to ensure product quality traceability.
Applications:
Drug Research and Development: Used for new drug screening, pharmacodynamics evaluation, and toxicology studies.
Production Monitoring: As a quality control standard in the production process to ensure product stability.
Regulatory Compliance: To meet the regulatory requirements of domestic and foreign drug regulatory agencies for drug impurity control.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $1160.00/1mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2024-12-20 | |
| $1160.00/1mg |
VIP3Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $1.00/1KG |
VIP7Y
|
Career Henan Chemical Co
|
2019-08-30 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake




 China
China