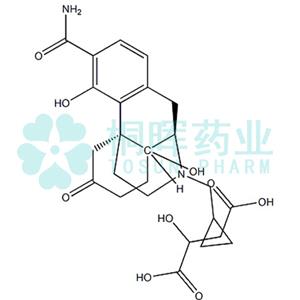
Samidorphan L-malate NEW
| Price | Get Latest Price | ||
| Package | 5kg | 10kg | 50kg |
| Min. Order: | 5kg |
| Supply Ability: | 200kg |
| Update Time: | 2024-12-30 |
Product Details
| Product Name: Samidorphan L-malate | CAS No.: 1204592-75-5 |
| EC-No.: 807-011-7 | Min. Order: 5kg |
| Purity: 98%-102% | Supply Ability: 200kg |
| Release date: 2024/12/30 | |
| MF: C25H33N2O9 | MW: 505.53752 |
Samidorphan L-malate API
Name:Samidorphan L-malate
CAS:1204592-75-5
MF:C25H33N2O9
MW:505.53752
EINECS:807-011-7
MDL No.:
Properties
InChIKeyLZSRIAXIOSOYRZ-AQELTAAUNA-N
SMILESC1C2=C([C@]34CCN(CC5CC5)[C@H](C2)C3(O)([H])CCC(=O)C4)C(O)=C(C(N)=O)C=1.C(O)(=O)C(CC(O)=O)O |&1:3,11,r|
Hot Tags: Samidorphan L-malate api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock
Samidorphan L-malate API pharmaceutical export
Samidorphan L-malate API pharmaceutical wholesaler
Shortage Samidorphan L-malate API Supplies
supply of various Samidorphan L-malate API
Samidorphan L-malate API Import and Export Wholesaler
wholesale distribution of Samidorphan L-malate API
Samidorphan L-malate API DISTRIBUTION CHANNELS
Wholesaler Distributor Licensure of Samidorphan L-malate API
Certificate of Analysis (COA) for Samidorphan L-malate API
Valid Samidorphan L-malate API GMP certification
Valid Samidorphan L-malate API Manufacturing License
Method of Analysis for Samidorphan L-malate API
Material safety data sheet for Samidorphan L-malate API
Accelerated stability data for Samidorphan L-malate API
Real time stability data for Samidorphan L-malate API
Samidorphan L-malate API Exporters and Contract manufacturing
Samidorphan L-malate in USP, BP, EP with DMF, Tech Pack, GMP, Written Confirmation
Regulatory Documents of Samidorphan L-malate
Samidorphan L-malate API of manufacturers who are USFDA audited & accredited with GMP, ISO
Samidorphan L-malate with DMF/GMP/ISO (all technical documents support)
Samidorphan L-malate API in USP/BP/EP pharmacopoeia
Samidorphan L-malate Micronised, Injection, & all grades
Samidorphan L-malate API factory accredited by USFDA, Health Canada, TGA, UK-MHRA, PIC/S
Samidorphan L-malate API with cGMP manufacturing facility、GMP certified facility
Samidorphan L-malate API with GLP certified laboratory
Samidorphan L-malate API with cGMP & WHO GMP compliant facility
Samidorphan L-malate API with R&D and Commercial quantity
Dosage form of Samidorphan L-malate API
Samidorphan L-malate API U.sPharmacopeia (UsP), in-house specification and/or European Pharmacopoeia (Ph. Eur)
Samidorphan L-malate API distributor with a strong supply chain channel
Samidorphan L-malate API Provide CEP/COS(certificate of suitability to monograph of European Pharmacopoeia) , EDMF(European Drug Master File),and GMP(Good Manufacturing Practice ),Written Confirmation.
Samidorphan L-malate API certificate of analysis
Samidorphan L-malate API factory GMP (Goods Manufacturing Practices Certificate )
Samidorphan L-malate API Product DML (Drug Manufacturing License)
TOSUN PHARM was established in 1999. In China, it is a collection of drugs, APIs, reference listed drugs, impurities, excipients, intermediates import and export, import registration services, generic drug research and development services, innovative drugs and high-end FDF technology transfer, A group company integrating marketing, academic promotion and cooperative production. With a global procurement, R&D and marketing network, it can quickly meet the needs of product R&D, production and market sales of Chinese and global pharmaceutical companies.
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP1Y
|
Moxin Chemicals
|
2024-12-31 | |
| $0.00/200kg |
VIP1Y
|
Qingdao RENAS Polymer Material Co., Ltd.
|
2024-08-26 | |
| $167.00/1mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 |
- Since: 1999-12-28
- Address: 12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China



 China
China