
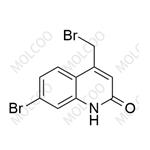
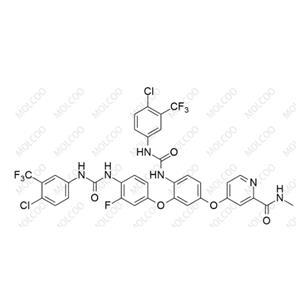
Rebamipide Impurity 26 NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 100000 |
| Update Time: | 2025-01-22 |
Product Details
| Product Name: Rebamipide Impurity 26 | CAS No.: 1451056-51-1 |
| Min. Order: 10mg | Purity: 98 |
| Supply Ability: 100000 | Release date: 2025/01/22 |
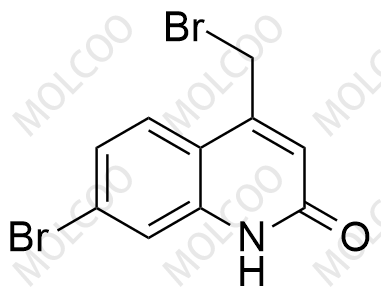
Rebamipide Impurity Standard
Product Name: Rebamipide Impurity Standard
English Name: Rebamipide Impurity Standard
Storage Conditions: It is recommended to store in a refrigerated environment at 2-8°C to maintain product stability.
Purity Grade: ≥98% (Determined by HPLC)
Product Category: Pharmaceutical Impurity Reference Standard
Product Details:
We are pleased to introduce our high-purity Rebamipide impurity standards, specifically designed to meet the stringent requirements of drug development, quality control, and drug registration fields. Utilizing advanced preparation techniques and rigorous quality control processes, we ensure that each batch meets or exceeds the industry-specified purity standards.
Our Rebamipide impurity standards are not only used for establishing and validating analytical methods but also play a crucial role in accurately assessing the impurity content in the main components of drugs, which is significant for ensuring drug safety and improving drug quality. Additionally, they are indispensable key materials in new drug registration applications.
We commit to providing comprehensive technical support and after-sales service, ensuring that any questions from customers during use can be promptly and professionally answered.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/25Kg/Bag |
VIP4Y
|
Sinoway Industrial co., ltd.
|
2021-07-21 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-23 | |
| $45.00/500mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake





 China
China