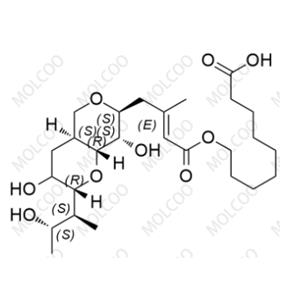
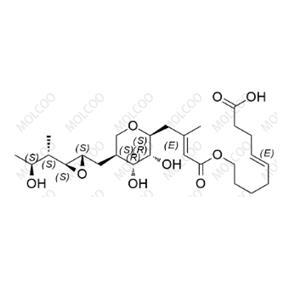
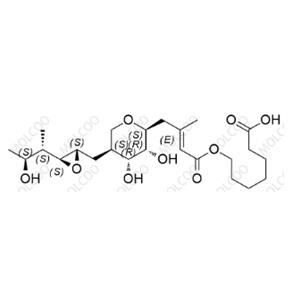
Mupirocin EP Impurity E NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 100000 |
| Update Time: | 2025-01-16 |
Product Details
| Product Name: Mupirocin EP Impurity E | CAS No.: 71087-96-2 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 100000 | Release date: 2025/01/16 |
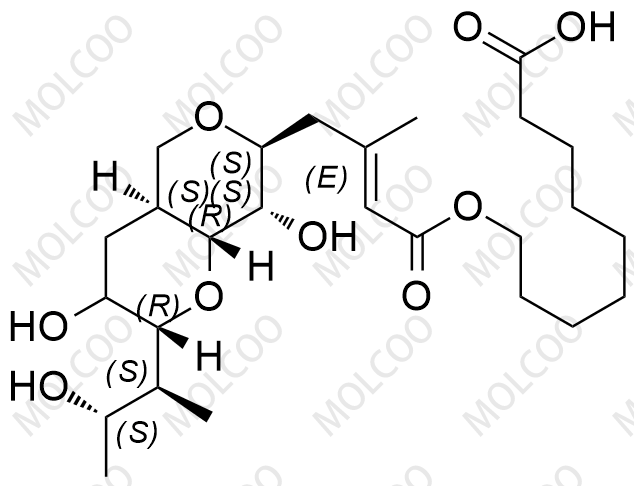
Product Name:Mupirocin Impurity Reference Standards
Product Overview:Mupirocin Impurity Reference Standards are reference materials specifically designed for drug research and development, quality control, and regulatory submissions. These impurity reference standards cover a wide range of impurities that may be present in Mupirocin drugs, including but not limited to EP Impurity A, B, C, D, E, etc. They are rigorously prepared and purified to ensure high purity and stability, accurately reflecting the impurity characteristics of Mupirocin drugs.
Application Areas:
Drug Research and Development:During the drug research and development phase, Mupirocin Impurity Reference Standards can be used to assess the purity and stability of new compounds, helping researchers identify and remove potential harmful impurities.
Quality Control:In the drug production process, using Mupirocin Impurity Reference Standards ensures that the impurity content of the product meets relevant regulations and standards, thereby improving product quality and safety.
Regulatory Submissions:During drug registration and approval processes, Mupirocin Impurity Reference Standards can serve as critical evidence demonstrating that the impurity content in the drug is within acceptable limits, facilitating the smooth approval of the drug.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2024-12-20 | |
| $999.00/1g |
VIP1Y
|
HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
|
2024-08-13 | |
| $29.00/10mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake





 China
China