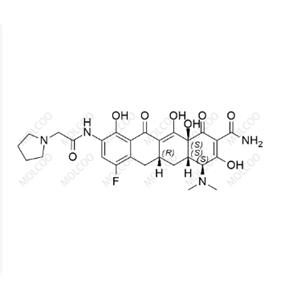
Product Details
| Product Name: Eravacycline | CAS No.: 1207283-85-9 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 1000000 | Release date: 2024/12/26 |

We have a complete set of impurities, all available in stock. Please contact us if you have any needs.

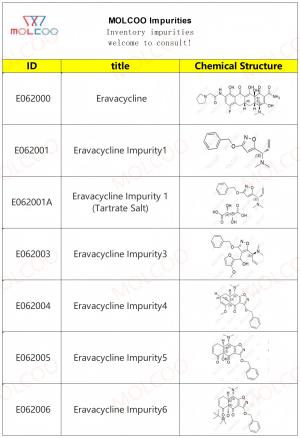
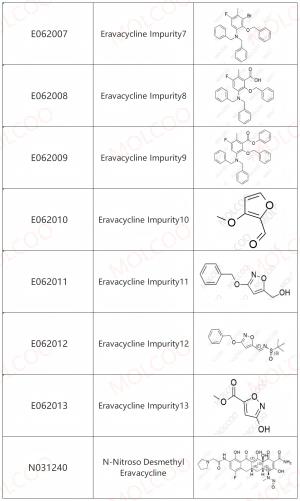
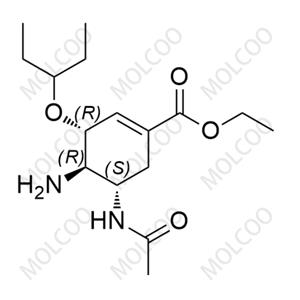
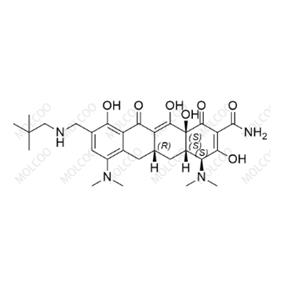
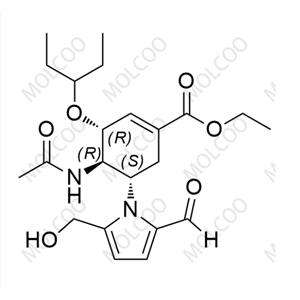
Eravacycline Impurity Reference Standards
Product Details:
Eravacycline impurity reference standards are crucial tools for detecting and verifying the purity and quality of eravacycline. These reference standards are produced according to strict standards and quality control procedures to ensure their accuracy and reliability.
Product Features:
High Purity: The purity of the impurity reference standards is above 95%, ensuring the accuracy of test results.
Multiple Specifications: Various specifications and packaging options are available to meet the needs of different customers.
Comprehensive Testing: Equipped with basic detection methods such as COA (Certificate of Analysis), HPLC (High-Performance Liquid Chromatography), H-NMR (Proton Nuclear Magnetic Resonance), and MS (Mass Spectrometry). Additional testing can be conducted based on customer requirements.
Excellent Service: Custom synthesis services are provided to meet the specific needs of customers.
Applications:
Eravacycline impurity reference standards are widely used in new drug research, drug registration, consistency evaluation, and drug quality control. They play a vital role in the new drug application and drug registration processes, helping to improve the purity and quality of drugs, thereby enhancing their efficacy and safety.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $6700.00/100mg |
VIP3Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $0.10/1KG |
VIP6Y
|
Shaanxi Dideu Medichem Co. Ltd
|
2024-08-04 | |
| $0.00/1kg |
VIP5Y
|
Hebei Yanxi Chemical Co., Ltd.
|
2023-08-16 | |
| $1.00/1KG |
VIP6Y
|
Career Henan Chemical Co
|
2020-01-01 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake





 China
China