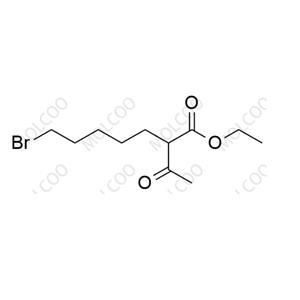
Product Details
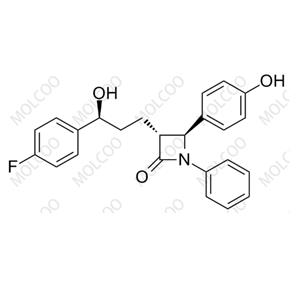
| Product Name: Cilastatin Impurity | CAS No.: 1114-50-7 |
| Min. Order: 10mg | Purity: 98 |
| Supply Ability: 100000000 | Release date: 2024/12/20 |
| Cilastatin: Cilastatin | Cilastatin: Cilastatin Impurity |
| Cilastatin: custom synthesis impurities |
We understand that impurities are not just about the purity of a drug; they directly relate to patient safety and efficacy. Therefore, we are dedicated to the research and control of Cilastatin impurities, utilizing advanced detection technologies to precisely identify and remove every trace of impurities that may compromise drug quality.
Through a rigorous quality control system, we ensure that each batch of Cilastatin meets the highest standards, providing patients with a safe, effective, and reliable treatment option. We firmly believe that only by having zero tolerance for impurities can we uphold the dignity and value of every life.


Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP1Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-04-15 | |
| $0.00/100mg |
VIP3Y
|
TargetMol Chemicals Inc.
|
2024-10-28 | |
| $1.00/1g |
VIP4Y
|
WUHAN FORTUNA CHEMICAL CO., LTD
|
2021-12-27 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake


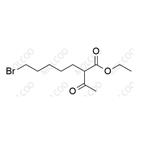



 China
China