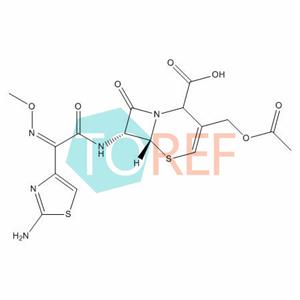
Cefotaxime Impurity K(Double Bond Transfer) NEW
| Price | Get Latest Price | ||
| Package | 5mg | 10mg | 50mg |
| Min. Order: | 5mg |
| Supply Ability: | 1000mg |
| Update Time: | 2024-12-25 |
Product Details
| Product Name: Cefotaxime Impurity K(Double Bond Transfer) | Min. Order: 5mg |
| Purity: 95%+ | Supply Ability: 1000mg |
| Release date: 2024/12/25 | |
| MF: C16H17N5O7S2 | MW: 455.46 |
Product Name:Cefotaxime Impurity K(Double Bond Transfer)
CAS No. N/A
Product Code:REF-C55021
MF:C16H17N5O7S2
MW: 455.46
Purity: 95%+
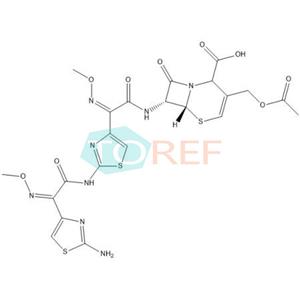
Chemical name:(6R,7R)-3-(acetoxymethyl)-7-((Z)-2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-3-ene-2-carboxylic acid
Storage conditions: 2-8 ℃
Synthesis of Cefotaxime Impurity K(Double Bond Transfer)
Cefotaxime Impurity K(Double Bond Transfer) Reference Standards,
Complex Impurity Of Cefotaxime Impurity K(Double Bond Transfer),
Chemical Standards of Cefotaxime Impurity K(Double Bond Transfer),
Characterization Of Unknown Impurities of Cefotaxime Impurity K(Double Bond Transfer),
Structure Profiling of Cefotaxime Impurity K(Double Bond Transfer)
Identification of Cefotaxime Impurity K(Double Bond Transfer)
Isolation & Purification of Impurity of Cefotaxime Impurity K(Double Bond Transfer)
Difficult of Cefotaxime Impurity K(Double Bond Transfer)
Instability of Cefotaxime Impurity K(Double Bond Transfer)
Low-assay of Cefotaxime Impurity K(Double Bond Transfer)
Complex Impurity Of Chemical Standards of Cefotaxime Impurity K(Double Bond Transfer)
TOSUN PHARM was established in 1999. In China, it is a collection of drugs, APIs, reference listed drugs, impurities, excipients, intermediates import and export, import registration services, generic drug research and development services, innovative drugs and high-end FDF technology transfer, A group company integrating marketing, academic promotion and cooperative production. With a global procurement, R&D and marketing network, it can quickly meet the needs of product R&D, production and market sales of Chinese and global pharmaceutical companies.
Hot Tags: Cefotaxime Impurity K(Double Bond Transfer), China, suppliers, manufacturers, factory, customized, price, pricelist, in stock
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP1Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-05-28 | |
| $0.00/10mg |
Guangzhou PI PI BIOTECH INC
|
2022-04-26 | ||
| $0.00/100mg |
VIP3Y
|
TargetMol Chemicals Inc.
|
2024-10-28 |
- Since: 1999-12-28
- Address: 12th floor, No. 181, Kexue Avenue, Huangpu District, Guangzhou, China



 China
China