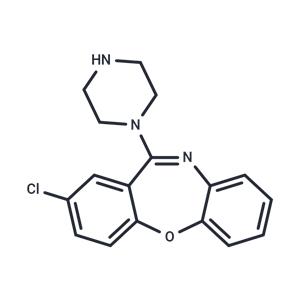
Amoxapine NEW
| Price | $64 |
| Package | 500mg |
| Min. Order: | |
| Supply Ability: | 10g |
| Update Time: | 2024-11-19 |
Product Details
| Product Name: Amoxapine | CAS No.: 14028-44-5 |
| Purity: 98.84% | Supply Ability: 10g |
| Release date: 2024/11/19 |
Product Introduction
Bioactivity
| Name | Amoxapine |
| Description | Amoxapine (CL-67772) exerts its antidepressant effect by inhibiting the re-uptake of norepinephrine and, to a lesser degree, of serotonin, at adrenergic nerve endings and blocks the response of dopamine receptors to dopamine. Amoxapine is a tricyclic antidepressant of the dibenzoxazepine class. This drug is used to treat symptoms of depression and may cause tardive dyskinesia. Amoxapine also binds to alpha-adrenergic, histaminergic, and cholinergic receptors which accounts for many of the side effects seen with this agent. |
| In vitro | Amoxapine, administered intraperitoneally (i.p.) at doses of 1, 5, and 10 mg/kg, notably at lower doses, was found to decrease paradoxical sleep and increase slow-wave sleep. Throughout the treatment period, a consistent reduction in paradoxical sleep was observed with Amoxapine (10 mg/kg, i.p.), although tolerance to the inhibition effect of cericlamine was noted in this sleep phase. Additionally, Amoxapine (10 mg/kg/day) did not affect the levels of Substance P, dynorphin, and cholecystokinin, but significantly increased leucine-enkephalin levels in the rat cortex, spinal cord, and hypothalamus. Despite not altering opioid receptor binding in the rat cortex, Amoxapine (10 mg/kg/day, i.p.) increased the density of δ- and μ-opioid receptor binding sites in the spinal cord and decreased it in the hypothalamus. Moreover, Amoxapine attenuated spontaneous activity, induced catalepsy and ptosis, and exhibited inhibitory effects on the dyskinetic movements induced by Apomorphine and stereotypy behaviors induced by amphetamine, through altering avoidance behaviors discerned by monkeys. |
| In vivo | In both oocytes and HEK 293 cells, Amoxapine induces acute hERG channel blockade with IC50 values of 21.6 and 5.1 μM, respectively. In human embryonic kidney 293 cells, it selectively inhibits GLYT2a over its isotype GLYT1b by a factor of 10. Amoxapine leads to a blockade of reverse frequency dependence and causes a leftward shift with accelerated inactivation. Treatment with Amoxapine results in a gradual reduction of hERG transport to the cell membrane surface in HEK 293 cells, with an IC50 of 15.3 μM. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 3.14 mg/mL (10 mM), Sonication is recommended. H2O : < 1 mg/mL (insoluble or slightly soluble) Ethanol : < 1 mg/mL (insoluble or slightly soluble) |
| Keywords | inhibit | Inhibitor | CL 67772 | Amoxapine | CL67772 |
| Inhibitors Related | Sarcosine | Opiranserin hydrochloride | Iclepertin | Org 25543 hydrochloride | Opiranserin | Bitopertin (R enantiomer) | LY2365109 hydrochloride | Bitopertin | PF-03463275 | ALX-1393 |
| Related Compound Libraries | FDA-Approved & Pharmacopeia Drug Library | Bioactive Compound Library | Anti-Neurodegenerative Disease Compound Library | Neuronal Signaling Compound Library | Drug Repurposing Compound Library | CNS-Penetrant Compound Library | Inhibitor Library | Anti-Cardiovascular Disease Compound Library | FDA-Approved Drug Library | Bioactive Compounds Library Max |
Company Profile Introduction
Target Molecule Corp. (TargetMol) is a global high-tech enterprise, headquartered in Boston, MA, specializing in chemical and biological research product and service to meet the research needs of global customers.
TargetMol has evolved into one of the biggest global compound library and small molecule suppliers and a customer based on 40+ countries. TargetMol offers over 80 types of compound libraries and a wide range of high-quality research chemicals including inhibitors, activator, natural compounds, peptides, inhibitory antibodies, and novel life-science kits, for laboratory and scientific use. Besides, virtual screening service is also available for customers who would like to conduct the computer-aided drug discovery.
You may like
- Since: 2011-01-07
- Address: 36?Washington?Street, Wellesley?Hills
INQUIRY



 United States
United States