
Arbekacin
| Price | $1 |
| Package | 1g |
| Min. Order: | 1g |
| Supply Ability: | g/kg/Ton |
| Update Time: | 2019-12-26 |
Product Details
| Product Name: Arbekacin | CAS No.: 51025-85-5 |
| Min. Order: 1g | Purity: ≥98% |
| Supply Ability: g/kg/Ton | Release date: 2019/12/26 |
| Delivery Time: Prompt | Packaging: Bottle/Durm/Bag |
| Transportation: By air/Bay Sea/Express |
Heidi 0656
Email:heidi@coreychem.com
▼
▲
Arbekacin Basic information
▼
▲
Product Name:
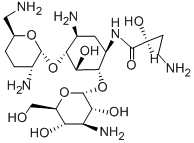
Arbekacin
Synonyms:
AHB[1KB;Blubatosine;(s)-oxy-1-oxobutyl)-2-deoxy;Decontasin;4-O-[3-Amino-3-deoxy-α-D-glucopyranosyl]-6-O-[2,6-diamino-2,3,4,6-tetradeoxy-α-D-erythro-hexopyranosyl]-N'-[(2S)-4-amino-2-hydroxy-1-oxobutyl]-2-deoxy-L-streptamine;6-O-(3-Amino-3-deoxy-α-D-glucopyranosyl)-4-O-(2,6-diamino-2,3,4,6-tetradeoxy-α-D-erythro-hexopyranosyl)-N1-[(S)-4-amino-2-hydroxybutyryl]-2-deoxy-D-streptamine;3,4,6-tetradeoxy-alpha-d-erythro-hexopyranosyl-(1-4))-n(sup1)-(4-amino-2-hydr;habekacin
CAS:
51025-85-5
MF:
C22H44N6O10
MW:
552.62
EINECS:
Product Categories:
Mol File:
51025-85-5.mol
▼
▲
Arbekacin Chemical Properties
▼
▲
Boiling point
904.0±65.0 °C(Predicted)
density
1.47±0.1 g/cm3(Predicted)
pka
13.07±0.70(Predicted)
CAS DataBase Reference
51025-85-5(CAS DataBase Reference)
▼
▲
Safety Information
▼
▲
▼
▲
MSDS Information
▼
▲
Arbekacin Usage And Synthesis
▼
▲
Description
Arbekacin is a semi-synthetic derivative of dibekacin useful in the treatment of bacterial infections. This aminoglycoside is active against a broad spectrum of bacteria, including some of the gentamycin-, kanamycin-, and tobramycin-resistant pathogens. Compared to amikacin and dibekacin, ototoxicity is reportedly milder.
Originator
Inst. Microbial Chemistry (Japan)
Definition
ChEBI: A kanamycin that is kanamycin B bearing an N-(2S)-4-amino-2-hydroxybutyryl group on the aminocyclitol ring.
Brand name
Habekacin
Antimicrobial activity
The 1-N-(4-amino-2-hydroxybutyryl) derivative of dibekacin, to which it bears the same relation as amikacin bears to kanamycin A. Supplied as the sulfate.
Activity and stability to aminoglycoside-modifying enzymes are comparable with those of amikacin. It is active against many strains of methicillin-resistant Staph. aureus, either alone or in combination with β-lactam or other agents. Synergy with ampicillin has been observed for high-level gentamicin- and vancomycin-resistant enterococci.
A 3 mg/kg intravenous dose achieved a peak concentration of c. 8 mg/L after 1 h. The plasma half-life is about 2 h and protein binding 3–12%.
About 85% of the dose can be recovered from urine over 48 h. It is retained in renal failure, but moderately well removed by hemodialysis with a plasma half-life of 2–4 h. Peak concentrations of 10.9 mg/L and trough concentrations of 1.7 mg/L have been reported in patients treated for MRSA infection where Cmax:MIC ratios of >25 and AUC:MIC ratios of >186 were associated with improved cure rates, and both Cmin and AUC were associated with the incidence of nephrotoxicity.
Toxicity and side effects are typical of the aminoglycoside class. It is used in severe infection cause by susceptible microorganisms, but is not widely available.
Activity and stability to aminoglycoside-modifying enzymes are comparable with those of amikacin. It is active against many strains of methicillin-resistant Staph. aureus, either alone or in combination with β-lactam or other agents. Synergy with ampicillin has been observed for high-level gentamicin- and vancomycin-resistant enterococci.
A 3 mg/kg intravenous dose achieved a peak concentration of c. 8 mg/L after 1 h. The plasma half-life is about 2 h and protein binding 3–12%.
About 85% of the dose can be recovered from urine over 48 h. It is retained in renal failure, but moderately well removed by hemodialysis with a plasma half-life of 2–4 h. Peak concentrations of 10.9 mg/L and trough concentrations of 1.7 mg/L have been reported in patients treated for MRSA infection where Cmax:MIC ratios of >25 and AUC:MIC ratios of >186 were associated with improved cure rates, and both Cmin and AUC were associated with the incidence of nephrotoxicity.
Toxicity and side effects are typical of the aminoglycoside class. It is used in severe infection cause by susceptible microorganisms, but is not widely available.
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $200.00/1kg |
Hebei Mingeng Biotechnology Co., Ltd
|
2022-11-20 |
- Since: 2014-12-17
- Address: 702, Building 10, East District, National University Science and Technology Park, High tech Zone, Zh
INQUIRY








 China
China