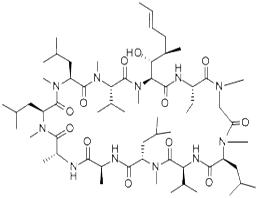
Cyclosporin A
| Price | $1 |
| Package | 1KG |
| Min. Order: | 1G |
| Supply Ability: | 100KG |
| Update Time: | 2019-07-06 |
Product Details
| Product Name: Cyclosporin A | CAS No.: 59865-13-3 |
| Min. Order: 1G | Purity: 98% |
| Supply Ability: 100KG | Release date: 2019/07/06 |
AD68
| Product Name: | Cyclosporin A |
| Synonyms: | ol27-400;s7481f1;sandimmun;sandimmune;ANTIBIOTIC S 7481F1;ANTIBIOTIC S 7481FI;CICLOSPORIN;CICLOSPORIN A |
| CAS: | 59865-13-3 |
| MF: | C62H111N11O12 |
| MW: | 1202.61 |
| EINECS: | 200-835-2 |
| Product Categories: | Active Pharmaceutical Ingredients;Organics;Intermediates & Fine Chemicals;Peptides;Pharmaceuticals;Protein Phosphatase;Signalling;KAYQUINONE;antibiotic;Anti-cancer&immunity;Inhibitors |
| Mol File: | 59865-13-3.mol |
 |
|
| Cyclosporin A Chemical Properties |
| Melting point | 148-151°C |
| alpha | D20 -244° (c = 0.6 in chloroform); D20 -189° (c = 0.5 in methanol) |
| Boiling point | 838.63°C (rough estimate) |
| density | 0.9913 (rough estimate) |
| refractive index | 1.6500 (estimate) |
| Fp | 87℃ |
| storage temp. | 2-8°C |
| solubility | ethanol: 30 mg/mL |
| form | solid |
| color | white |
| Water Solubility | Soluble in dimethyl sulfoxide and ethanol. Insoluble in water. |
| Merck | 14,2752 |
| CAS DataBase Reference | 59865-13-3(CAS DataBase Reference) |
| EPA Substance Registry System | Cyclosporin A(59865-13-3) |
| Safety Information |
| Hazard Codes | T,Xn,F |
| Risk Statements | 45-60-22-40-36-20/21/22-11 |
| Safety Statements | 53-45-36/37-24/25-22-26-16 |
| RIDADR | UN 1648 3 / PGII |
| WGK Germany | 3 |
| RTECS | GZ4120000 |
| Hazardous Substances Data | 59865-13-3(Hazardous Substances Data) |
| Toxicity | LD50 in mice, rats, rabbits (mg/kg): 107, 25, >10 i.v.; 2329, 1480, >1000 orally (Ryffel) |
| Cyclosporin A Usage And Synthesis |
| Immunosuppressants | Cyclosporin A is a lipophilic cyclic peptide compounds separated by certain Hyphomycetes fungi, such as Cylindrocarpon lucidum and Tolypocladium inflatumculture medium. In 1980s, as an immunosuppressant, it has been widely used in the world, is now used for a variety of tissues and organ transplant to prevent rejection reaction and to treat autoimmune diseases. Cyclosporin A is a strong immunosuppressant, inhibits the onset of the reaction in cell media, reversibly inhibits T cell proliferation, does not inhibit the generation of blood cells, does not affect the function of the phagocyte. As compared with other inhibitors, fewer advantages are induce or aggravate infection, mainly used in clinical kidney, liver, heart, lung, bone marrow transplant anti-rejection, can be combined with adrenal corticosteroids, but not in combination with other immunosuppressive agents. Also used in certain autoimmune diseases (such as systemic lupus erythematosus, lupus nephritis, rheumatoid arthritis, autoimmune hemolytic anemia). Try to uveitis, severe aplastic anemia, refractory idiopathic thrombocytopenic purpura, psoriasis, etc. |
| Pharmacological effects | Cyclosporin A can reversibly selectively alter T-lymphocyte function, prevent transcription of lymphokine gene, interfere transfer of original information, inhibit the release of interleukin-2, interferon and other immune factors, affect humoral immunity and immune cells, inhibit killing viability of NK cell; inhibit differentiation and proliferation of lymphocyte in the antigen or mitogen stimulation. But no activity on bone marrow, very small effect on B cells, at therapeutic doses no significant inhibiting effect on granulocyte survival. Oral absorption is slow and incomplete, only 4% to 60%, an average is 30% of the administered dose; relative bioavailability is 20% to 50%. After 2~8h of oral administration reach its peak, with an average of 3.5h. Significant individual differences, such as the relative bioavailability of renal transplant patients is 5% to 89%, liver transplant patients is 8% to 60%. This product is fat-soluble drug, meals and eating food can increase the absorption of fat. Bile deficiency, stagnation, slow gastric emptying, increased gastric peristalsis, pancreatic dysfunction and steatorrhea, all affect absorption of the product. |
| Pharmacokinetics | Oral absorption is irregular and big differences in different individual, bioavailability is 30%, but can be increased with the extended duration of treatment and the increased dose. After liver transplantation, patients with liver disease or disorder of the gastrointestinal absorption may be reduced . Binding rate this product with plasma proteins is up to about 90%, mainly in combination with lipoproteins. Oral peak time is 3 to 4 hours, whole blood concentration is 2 to 9 times of plasma, adult plasma T1/2 is 19 (10-27) hours, while the child is only 7 (7 to 19) hours. This product is metabolized in the liver, by biliary excretion through feces, only 6% by renal excretion, of which about 0.1% is excreted in the primary form. The above information is edited by the Chemicalbook of Liu Yujie. |
| Indications | For prevention of a variety of tissue and organ transplant rejection and treatment of some autoimmune diseases, also used in kidney disease, aplastic anemia, refractory ulcerative colitis. In dermatology, for stubborn and intractable skin diseases, the best effect is severe psoriasis (including vulgaris, pustular, erythrodermic and joint disease); good efficacy on skin disorders including Behcet disease, gangrenous pyoderma, lichen planus, acquired epidermolysis bullosa psychosis; moderate efficacy on disease including alopecia, atopic dermatitis, chronic actinic dermatitis, light class of reticulocyte cell histiocytosis, adult linear IgA bullous dermatitis, pemphigus, bullous pemphigoid (the latter two illness should be in combination with corticosteroids). In addition, Cyclosporine A has effect on systemic lupus erythematosus, dermatomyositis, systemic sclerosis, AIDS, ichthyosis, cutaneous T-cell lymphoma, pityriasis rubra pilaris, generalized missed acrodermatitis, palmoplantar pus blister disease, male pattern baldness, vitiligo, progressive necrotizing xanthogranulomatous, generalized granuloma annulare, lichen myxedema, papular mucin calm disease, hyperthyroidism. |
| Drug interactions | 1, In addition to corticosteroids, this product should be avoided in combination with any other immunosuppressive agents. 2, The inactivation of product is mainly in the liver metabolic, any drugs which can affect the liver enzyme activities can affect the metabolism of the product, such as erythromycin, josamycin, doxycycline, ketoconazole, H2 receptor antagonists , calcium antagonists, androgens, oral contraceptives affect activity of cytochrome P-450 liver cell, increase the risk of toxicity of this product. 3, Carbamazepine, phenytoin, phenobarbital, isoniazid, rifampin, et al. can make the plasma concentration of this product decrease. 4, Aminoglycoside antibiotic, trimethoprim-sulfamethoxazole, TMP, amphotericin B, cephalosporins, mechlorethamine, nonsteroidal anti-inflammatory drug, mannitol, furosemide, et al increase nephrotoxicity of cyclosporine . 5, Calcium and increased calcium are disabled when using this product, and cannot be vaccinated. 6, Long-term in combination with glucocorticoid can trigger diabetes, hypertension, ulcer disease and osteoporosis, and can increase the toxicity of this product. 7, Before used Cyclosporin A, other immunosuppressive agents are used, patients have overall decreased immunity and are easy to be infected. |
| Side effects | 1, Kidney toxicity: accounted for about 13%, may have glomerular thrombosis, tubular obstruction, mitochondrial swelling, proteinuria, tubular urine; occasionally hyperuricemia, hyperkalemia, elevated serum creatinine, blood urea nitrogen rise, little or no urine. 2, Liver toxicity: hypoproteinemia, hyperbilirubinemia, serum alkaline phosphatase and lactate dehydrogenase elevated, when the serum concentration of the drug is greater than 200ng/ml most likely to occur, especially in the original hepatitis or liver dysfunction. 3, Nervous system: usually presents exercise-induced spinal cord syndrome, cerebellar ataxia, mental confusion, tremors, abnormal feeling, etc. 4, Gastrointestinal reactions: common anorexia, nausea, and vomiting. Other reactive hypertension, hirsutism. When administered intravenously, rare serious allergic reactions such as chest facial flushing, breathing difficulties, asthma, heart palpitations; once presents the above symptoms, should be discontinued immediately and anti-shock first aid. |
| Chemical properties | From-15 ℃ of acetone to obtain white needle crystallization, melting point 148~151 ℃, [α] D20-244 ° (C = 0.6, chloroform). Soluble in methanol, ethanol, acetone, ether or chloroform, slightly soluble in water and saturated hydrocarbons. Acute toxicity LD50 mouse, rat, rabbit (mg/kg): 107, 25, > 10 intravenous injection; 2329,1480,> 1000 oral. |
| Production methods | Used Tolypocladium inflatum Gams as production strains. 50L seed medium is added into 75 L tank, inoculated with 5 × 109 spores, Ph = 5.4-4.3, cultured for 72 h, to obtain first order seed liquid. 500L fermentation medium is added into 750 L fermentation tank, inoculated with above first order seed liquid, cultured for 6 days to give second order seed liquid. 3000L fermentation medium is added into 4500L fermentation tank, fermentation for 12 days to give fermented cyclosporine toxicity. An equal volume of ethyl acetate is added into the fermentation broth, organic layer was separated, evaporated under reduced pressure to give the crude product. Melting process, can obtain Cyclosporin A and Cyclosporin C component, wherein Cyclosporin A production is about 150~200 mg/L, Cyclosporin C is about 50~100 mg/L. |
| Chemical Properties | White Powder |
| Uses | prothrombogenic agent |
| Uses | Cyclosporin A is a hydrophobic cyclic peptide isolated from several fungal species including Cylindrocarpon, Fusarium, Trichoderma and Tolypocladium. Cyclosporin A inhibits T-cell activation and has been marketed since 1983 as an immunosuppressant in post-allogeneic organ transplant. Cyclosporin A acts by binding to the protein, cyclophilin (immunophilin), in T-lymphocytes causing inhibition of calcineurin (protein phosphatase 2B). Cyclosporin A reduces transcription of interleukin 2, and inhibits lymphokine production, interleukin release and NO synthesis induced by interleukin 1α, lipopolysaccharides and TNFα. |
| Uses | An immunosuppressant that has revolutionized organ transplantation through its use in the prevention of graft rejection. A group of nonpolar cyclic oligopeptides with immunosupppressant activity. |
| Definition | ChEBI: A cyclic nonribosomal peptide of eleven amino acids; an immunosuppressant drug widely used in post-allogeneic organ transplant to reduce the activity of the patient's immune system, and therefore the risk of organ rejection. Also causes reversible inhibiti n of immunocompetent lymphocytes in the G0- and G1-phase of the cell cycle. |
| General Description | White prismatic needles (from acetone) or white powder. |
| Air & Water Reactions | Slightly water soluble . |
| Reactivity Profile | Cyclosporin A is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). |
| Health Hazard | SYMPTOMS: Symptoms of exposure to Cyclosporin A include hepatotoxicity, nephrotoxicity, hyperkalemia, hyperuricemia, convulsions, renal dysfunction, tremor, hirsutism, hypertension, gum hyperplasia, cramps, acne, headache, diarrhea, nausea, vomiting, abdominal discomfort, paresthesia, flushing, leukopenia, lymphoma, sinusitis and gynecomastia. In 2% or less of persons exposed, it has caused allergic reactions, anemia, anorexia, confusion, conjunctivitis, edema, fever, brittle fingernails, gastritis, hearing loss, hiccups, hyperglycemia, muscle pain, peptic ulcer, thrombocytopenia and tinnitus. Rare reactions include anxiety, chest pain, constipation, depression, hair breaking hematuria, joint pain, lethargy, mouth sores, myocardial infarction, night sweats, pancreatitis, pruritus, swallowing difficulty, tingling, upper gastrointestinal bleeding, visual disturbance, weakness and weight loss. It has caused kidney and liver damage. An increased susceptibility to infection may occur. Other symptoms include gastrointestinal disturbance, rashes and angioedema. |
| Fire Hazard | Flash point data for Cyclosporin A are not available; however, Cyclosporin A is probably combustible. |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals.
Mainly deals in the sales of:
Pharmaceutical intermediates
OLED intermediates:
Pharmaceutical intermediates;
OLED intermediates;
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $50.00/1KG |
VIP4Y
|
Hebei Lingding Biotechnology Co., Ltd.
|
2024-12-13 | |
| $50.00/100mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $50.00/100mg |
VIP3Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $999.00/5kg |
VIP1Y
|
HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
|
2024-08-20 | |
| $0.00/1kg |
VIP1Y
|
Shaanxi TNJONE Pharmaceutical Co., Ltd
|
2024-04-19 | |
| $9.00/10g |
VIP1Y
|
Hebei Kangcang new material Technology Co., LTD
|
2024-03-27 | |
| $0.00/25kg |
Shaanxi Haibo Biotechnology Co., Ltd
|
2023-09-04 | ||
| $50.00/1kg |
Henan Bao Enluo International TradeCo.,LTD
|
2023-06-29 | ||
| $0.00/20Gram |
VIP3Y
|
Wuhan Senwayer Century Chemical Co.,Ltd
|
2023-03-09 | |
| $0.00/1KG |
VIP3Y
|
Hebei Weibang Biotechnology Co., Ltd
|
2022-09-30 |
- Since: 2014-12-17
- Address: No.967,15th Floor,Unit 7, Building 1, No.70 of DianChang Road, High-tech Development Zone, Zhengzho
INQUIRY
楊俊青
sales@coreychem.com
sales@coreychem.com




 China
China