- Zenarestat
-
- $1820.00 / 100mg
-
2024-10-28
- CAS:112733-06-9
- Min. Order:
- Purity:
- Supply Ability: 10g
|
| | Zenarestat Basic information |
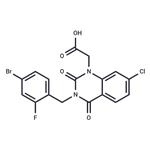
| Product Name: | Zenarestat | | Synonyms: | 3-(2-Fluoro-4-bromobenzyl)-7-chloro-2,4(1H,3H)-dioxoquinazoline-1-acetic acid;3-[(4-Bromo-2-fluorophenyl)methyl]-7-chloro-3,4-dihydro-2,4-dioxo-1(2H)-quinazolineacetic acid;FK-366;Zenarestat;FR 74366;2-[3-[(4-bromo-2-fluorophenyl)methyl]-7-chloro-2,4-dioxoquinazolin-1-yl]acetic acid;[3-(4-Bromo-2-fluorobenzyl)-7-chloro-2,4-dioxo-3,4-dihydro-1(2H)- quinazolinyl]acetic acid;1(2H)-Quinazolineacetic acid, 3-[(4-bromo-2-fluorophenyl)methyl]-7-chloro-3,4-dihydro-2,4-dioxo- | | CAS: | 112733-06-9 | | MF: | C17H11BrClFN2O4 | | MW: | 441.639 | | EINECS: | | | Product Categories: | | | Mol File: | 112733-06-9.mol | 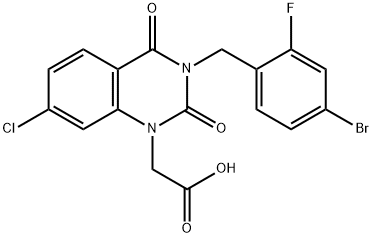 |
| | Zenarestat Chemical Properties |
| Melting point | 223-224° | | Boiling point | 624.4±65.0 °C(Predicted) | | density | 1.737 | | solubility | DMSO : 100 mg/mL (226.43 mM; Need ultrasonic) | | pka | 3.76±0.10(Predicted) | | form | Solid | | color | White to off-white |
| | Zenarestat Usage And Synthesis |
| Originator | FK 366 ,Fujisawa Pharmaceutical | | Uses | Treatment of diabetic neuropathy (aldose reductase inhibitor). | | Manufacturing Process | To a solution of 4-bromo-2-fluorobenzylamine and triethylamine in chloroform
was added dropwise a solution of 4-chloro-2-nitrobenzoyl chloride in
chloroform at 0°C with stirring and the mixture was stirred at the same
temperature for 1 h. The reaction mixture was washed in turn with diluted
aqueous hydrochloric acid and water, and then dried. Evaporation of the
solvent followed by recrystallization from diethyl ether gave N-(4-bromo-2-
fluorobenzyl)-4-chloro-2-nitrobenzamide.
A mixture of N-(4-bromo-2-fluorobenzyl)-4-chloro-2-nitrobenzamide and iron
(1.45 g) in acetic acid (66 ml) was stirred at 100°C for 30 min. After cooling,
iron was filtered off. The filtrate was evaporated to give a residue, which was
made alkaline with aqueous 1 N sodium hydroxide and extracted with ethyl
acetate. The extract was washed with water and dried. Removal of the solvent
gave 2-amino-N-(4-bromo-2-fluorobenzyl)-4-chlorobenzamide.
2-Amino-N-(4-bromo-2-fluorobenzyl)-4-chlorobenzamide and N,N'-
carbonyldiimidazole were dissolved in dioxane (50 ml). The solution was
evaporated to give a residue, which was stirred at 150°C for 30 min. After
cooling, the precipitates were collected by filtration and washed with ethanol
to give 3-(4-bromo-2-fluorobenzyl)-7-chloro-1,2,3,4-tetrahydro-2,4-
dioxoquinazoline; melting point >280°C.
To a suspension of 3-(4-bromo-2-fluorobenzyl)-7-chloro-1,2,3,4-tetrahydro-
2,4-dioxoquinazoline in N,N-dimethylformamide was added sodium hydride
(60% in mineral oil) with stirring at 0°C and the mixture was stirred for 15
min at the same temperature. To this mixture was added ethyl bromoacetate
and the mixture was stirred for 1 h at room temperature. The reaction
mixture was poured into diluted hydrochloric acid and extracted with ethyl
acetate. The extract was washed with brine, dried and evaporated to give a
residue. Thus obtained product was purified by recrystallization from isopropyl
ether to give 2-[3-(4-bromo-2-fluorobenzyl)-7-chloro-1,2,3,4-tetrahydro-2,4-
dioxoquinazolin-1-yl]acetic acid melting point 223°-224°C. | | Therapeutic Function | Aldose reductase inhibitor |
| | Zenarestat Preparation Products And Raw materials |
|