Lead acetate
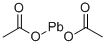
|
- $22 - $305
- Product name: Lead acetate
- CAS: 301-04-2
- MF: C4H6O4Pb
- MW: 325.29
- EINECS:206-104-4
- MDL Number:MFCD00012452
- Synonyms:ACETIC ACID, LEAD SALT TRIHYDRATE;LeadAcetateBasicAnhydrous;Leadacetateanhydrous;Diacetic acid lead(II) salt;Lead(II) diacetate;Lead(II)diacetate;Lead(2+) diacetate;LEAD(II) ACETATE 3-HYDRATE
5 prices
Selected condition:
Brand
- TCI Chemical
- TRC
- Usbiological
Package
- 1G
- 5G
- 25G
- ManufacturerTCI Chemical
- Product numberL0315
- Product descriptionLead(II) Acetate [for Perovskite precursor]
- Packaging1G
- Price$22
- Updated2024-03-01
- Buy
- ManufacturerTCI Chemical
- Product numberL0315
- Product descriptionLead(II) Acetate [for Perovskite precursor]
- Packaging5G
- Price$55
- Updated2024-03-01
- Buy
- ManufacturerTCI Chemical
- Product numberL0315
- Product descriptionLead(II) Acetate [for Perovskite precursor]
- Packaging25G
- Price$166
- Updated2024-03-01
- Buy
- ManufacturerTRC
- Product numberL320010
- Product descriptionLeadDiacetate
- Packaging25g
- Price$165
- Updated2021-12-16
- Buy
- ManufacturerUsbiological
- Product number453596
- Product descriptionLead Diacetate
- Packaging5g
- Price$305
- Updated2021-12-16
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| TCI Chemical | L0315 | Lead(II) Acetate [for Perovskite precursor] | 1G | $22 | 2024-03-01 | Buy |
| TCI Chemical | L0315 | Lead(II) Acetate [for Perovskite precursor] | 5G | $55 | 2024-03-01 | Buy |
| TCI Chemical | L0315 | Lead(II) Acetate [for Perovskite precursor] | 25G | $166 | 2024-03-01 | Buy |
| TRC | L320010 | LeadDiacetate | 25g | $165 | 2021-12-16 | Buy |
| Usbiological | 453596 | Lead Diacetate | 5g | $305 | 2021-12-16 | Buy |
Properties
Melting point :75 °C (dec.)(lit.)
Boiling point :decomposes at >280℃ [KIR78]
Density :3.3 g/cm3
vapor pressure :15.7hPa at 25℃
storage temp. :2-8°C
solubility :DMSO (Slightly), Methanol (Slightly)
form :Liquid
color :Clear colorless
Water Solubility :g/100g H2O: 19.7 (0°C), 55.2 (25°C); equilibrium solid phase, Pb(CH3COO)2 ·3H2O [KRU93]; g/100mL H2O: 44.3 (20°C), 221 (50°C) [KIR78]
λmax :260nm(H2O)(lit.)
Merck :14,5397
Solubility Product Constant (Ksp) :pKsp: 2.75
Dielectric constant :2.5(0.0℃)
LogP :-0.17
CAS DataBase Reference :301-04-2(CAS DataBase Reference)
EPA Substance Registry System :Lead(II) acetate (301-04-2)
Boiling point :decomposes at >280℃ [KIR78]
Density :3.3 g/cm3
vapor pressure :15.7hPa at 25℃
storage temp. :2-8°C
solubility :DMSO (Slightly), Methanol (Slightly)
form :Liquid
color :Clear colorless
Water Solubility :g/100g H2O: 19.7 (0°C), 55.2 (25°C); equilibrium solid phase, Pb(CH3COO)2 ·3H2O [KRU93]; g/100mL H2O: 44.3 (20°C), 221 (50°C) [KIR78]
λmax :260nm(H2O)(lit.)
Merck :14,5397
Solubility Product Constant (Ksp) :pKsp: 2.75
Dielectric constant :2.5(0.0℃)
LogP :-0.17
CAS DataBase Reference :301-04-2(CAS DataBase Reference)
EPA Substance Registry System :Lead(II) acetate (301-04-2)
Safety Information
| Symbol(GHS): |
 
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Danger | |||||||||||||||||||||
| Hazard statements: |
|
|||||||||||||||||||||
| Precautionary statements: |
|
Description
Lead acetate is stable under ordinary conditions of use and storage. Lead acetate is incompatible with bromates, phenol, chloral hydrate, sulphides, hydrogen peroxide, resorcinol, salicylic acid, sulphites, vegetable infusions, alkalis, tannin, phosphates, citrates, chlorides, carbonates, tartrates, and acids. Lead (II) acetate, as well as white lead, has been used in cosmetics throughout history, though this practice has ceased in Western countries. It is still used in men’s hair colouring. Lead (II) acetate paper is used to detect the poisonous gas hydrogen sulphide. The gas reacts with lead (II) acetate on the moistened test paper to form a grey precipitate of lead (II) sulphide.More related product prices
Lead acetate basic LEAD ACETATE Lead tetraacetate Lead acetate trihydrate LeadRelated product price
- Lead(II) salicylate
$455-7494.8 - LEAD SUBACETATE
$15-6501.5 - Lead citrate
$52-146
Suppliers and manufacturers
Shenyang Simchoice Chemical Co.,Ltd
Hefei TNJ Chemical Industry Co.,Ltd.
Hubei xin bonus chemical co. LTD
Chongqing Chemdad Co., Ltd
Richest Group Ltd
Antai Fine Chemical Technology Co.,Limited
Hefei TNJ Chemical Industry Co.,Ltd.





