Calcium carbide
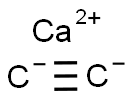
|
- $59.3 - $6700.45
- Product name: Calcium carbide
- CAS: 75-20-7
- MF: C2Ca
- MW: 64.1
- EINECS:200-848-3
- MDL Number:MFCD00010905
- Synonyms:carburedecalcium(french) ;carburocalcico ;calciumcarbide(cac2) ;calciumdicarbide ;carburedecalcium ;Calciumcarbid;Calcium Carbide, Anhydrous;Calcium Carbide, 10-40 Mesh
7 prices
Selected condition:
Brand
- Alfa Aesar
- American Custom Chemicals Corporation
- Sigma-Aldrich
Package
- 100G
- 500g
- 1kg
- 2kg
- *5x1kg
- ManufacturerAlfa Aesar
- Product number014308
- Product descriptionCalcium carbide
- Packaging1kg
- Price$63.5
- Updated2024-03-01
- Buy
- ManufacturerAlfa Aesar
- Product number014308
- Product descriptionCalcium carbide
- Packaging*5x1kg
- Price$250
- Updated2023-06-20
- Buy
- ManufacturerAmerican Custom Chemicals Corporation
- Product numberING0003887
- Product descriptionCALCIUM CARBIDE 95.00%
- Packaging1KG
- Price$6700.45
- Updated2021-12-16
- Buy
- ManufacturerSigma-Aldrich
- Product number270296
- Product descriptionCalcium carbide pieces, thickness <10 mm , typically, technical grade, ~80%
- Packaging500g
- Price$59.3
- Updated2024-03-01
- Buy
- ManufacturerSigma-Aldrich
- Product number21039
- Product descriptionCalcium carbide granulated, technical, ≥75% (gas-volumetric)
- Packaging100G
- Price$69.5
- Updated2024-03-01
- Buy
- ManufacturerSigma-Aldrich
- Product number270296
- Product descriptionCalcium carbide pieces, thickness <10 mm , typically, technical grade, ~80%
- Packaging2kg
- Price$123
- Updated2024-03-01
- Buy
- ManufacturerSigma-Aldrich
- Product number21039
- Product descriptionCalcium carbide granulated, technical, ≥75% (gas-volumetric)
- Packaging1KG
- Price$136
- Updated2024-03-01
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| Alfa Aesar | 014308 | Calcium carbide | 1kg | $63.5 | 2024-03-01 | Buy |
| Alfa Aesar | 014308 | Calcium carbide | *5x1kg | $250 | 2023-06-20 | Buy |
| American Custom Chemicals Corporation | ING0003887 | CALCIUM CARBIDE 95.00% | 1KG | $6700.45 | 2021-12-16 | Buy |
| Sigma-Aldrich | 270296 | Calcium carbide pieces, thickness <10 mm , typically, technical grade, ~80% | 500g | $59.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 21039 | Calcium carbide granulated, technical, ≥75% (gas-volumetric) | 100G | $69.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 270296 | Calcium carbide pieces, thickness <10 mm , typically, technical grade, ~80% | 2kg | $123 | 2024-03-01 | Buy |
| Sigma-Aldrich | 21039 | Calcium carbide granulated, technical, ≥75% (gas-volumetric) | 1KG | $136 | 2024-03-01 | Buy |
Properties
Melting point :447°C
Boiling point :2300°C
Density :2.22 g/mL at 25 °C(lit.)
storage temp. :water-free area
solubility :reacts with H2O
form :pieces
Specific Gravity :2.22
color :Gray-black
Water Solubility :hydrolyses
Sensitive :Moisture Sensitive
Merck :14,1656
BRN :3909011
Dielectric constant :5.8 - 7.0(0.0℃)
Exposure limits :ACGIH: TWA 2 mg/m3
OSHA: TWA 5 mg/m3
NIOSH: IDLH 25 mg/m3; TWA 2 mg/m3 Stability :Stability Reacts violently with water liberating highly flammable gas (acetylene). Do not use water if this material is involved in a fire. Incompatible with moisture, water, strong oxidizing agents, alcohols, hydrogen chloride, magnesium.
InChIKey :UIXRSLJINYRGFQ-UHFFFAOYSA-N
EPA Substance Registry System :Calcium carbide (75-20-7)
Boiling point :2300°C
Density :2.22 g/mL at 25 °C(lit.)
storage temp. :water-free area
solubility :reacts with H2O
form :pieces
Specific Gravity :2.22
color :Gray-black
Water Solubility :hydrolyses
Sensitive :Moisture Sensitive
Merck :14,1656
BRN :3909011
Dielectric constant :5.8 - 7.0(0.0℃)
Exposure limits :ACGIH: TWA 2 mg/m3
OSHA: TWA 5 mg/m3
NIOSH: IDLH 25 mg/m3; TWA 2 mg/m3 Stability :Stability Reacts violently with water liberating highly flammable gas (acetylene). Do not use water if this material is involved in a fire. Incompatible with moisture, water, strong oxidizing agents, alcohols, hydrogen chloride, magnesium.
InChIKey :UIXRSLJINYRGFQ-UHFFFAOYSA-N
EPA Substance Registry System :Calcium carbide (75-20-7)
Safety Information
| Symbol(GHS): |
  
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Danger | |||||||||||||||||||||||||||||||||||
| Hazard statements: |
|
|||||||||||||||||||||||||||||||||||
| Precautionary statements: |
|
Description
Calcium carbide (molecule formula: CaC2), is a kind of important chemical raw materials produced from the chemical processing of limestone. In 1892, H. Maysan (French) and H. Wilson (United state) simultaneously developed a calcium carbide production approach based on furnace Reduction. The United State had successfully achieved industrial production in 1895. The property of calcium carbide is related to its purity. Its industrial product is mostly the mixture of calcium carbide and calcium oxide, and also contains trace amounts of sulfur, phosphorus, nitrogen and other impurities. With the increasing content of impurities, it color exhibits gray, brown to black. The melting point and electrical conductivity both decrease with the decrease of the purity. The purity of its industrial product is usually 80% with m.p. being 1800~2000 °C. At room temperature, it does not react with air, but it can have oxidation reaction at above 350 ℃, and have reaction with nitrogen at 600~700 ℃ to generate calcium cyanamide. Calcium carbide, when coming across with water or steam, generates acetylene and release a large amount of heating. CaC2 + 2H2O─ → C2H2 + Ca (OH) 2 + 125185.32J, 1kg of pure calcium carbide can produce 366 L of acetylene 366l (15 ℃, 0.1MPa). Thereby, for its storage: calcium carbide should be strictly kept away from water. It is usually packed in a sealed iron container, and sometimes stored in a dry warehouse being filled with nitrogen if necessary.Electric furnace reduction method is the only method for industrial production of calcium carbide at present. Put calcium oxide and coke for reduction reaction at 2000~2200 ℃: CaO + 3C─ → CaC2 + CO-480644.64J, the resulting molten calcium carbide flow into the receiver tank from the bottom of the reactor, and we obtain the final product after cooling. Calcium carbide production belongs to high temperature operation with relative large amount dust being produced and consuming a large amount of electrical energy. In 1980s, the production of per ton of calcium carbide consumes industrial power of about 10~11GJ. In order to reduce the power consumption, people mostly apply large-scale and closed calcium carbide furnace to reduce heat loss and also do good to the recycling of carbon monoxide.
Calcium carbide is mainly used as the raw material for the production of acetylene. Acetylene is an important and basic organic chemical raw material. But with the rise of the petrochemical industry, organic chemicals supplied acetylene has gradually provided by petrochemicals. The acetylene generator of metal cutting and welding applies calcium carbide. In places without electricity power, people usually apply calcium carbide lamp as the light source. Industrially, people apply calcium carbide for being heated in ammonia to produce calcium cyanamide, which is a kind of nitrogen fertilizer, plant defoliants and herbicides. With the development of acetylene chemical industry, the China's production of calcium carbide increases from the 3000 tons in 1949 to 1.808 million tons in 1983, ranking first in the world. But the production technology is still relatively lagged behind. The majority are small open furnace with high energy consumption rate and serious pollution. In order to reduce the energy consumption, modern production of calcium carbide mostly applied closed large-scale furnace to reduce heat loss and facilitate the recycling of carbon monoxide.
The above information is edited by the chemicalbook of Dai Xiongfeng.
More related product prices
TUNGSTEN CARBIDE ALUMINUM CARBIDE Hydroxyapatite ALUMINUM OXIDE Calcium nitrate Sulfur dioxide HYDROXYLAPATITE Calcium fluoride ALUMINUM OXIDE Potassium superoxide Nitric acid Ammonium carbonate Barium carbonate Boron carbide SODIUM OXIDE Calcium CALCIUM HYDRIDE Calcium carbonateRelated product price
- TUNGSTEN CARBIDE
$37-757 - ALUMINUM CARBIDE
$217-4707.9 - Hydroxyapatite
$26-4474.44
Suppliers and manufacturers
Henan Tianfu Chemical Co.,Ltd.
Xiamen AmoyChem Co., Ltd
Hubei xin bonus chemical co. LTD
Shanghai Longyu Biotechnology Co., Ltd.
SIMAGCHEM CORP





