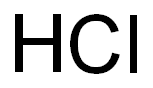Hydrochloric acid suppliers
Hydrochloric acid
- CAS:
- 7647-01-0
- MF:
- ClH
- MW:
- 36.46
Properties
- Melting point:
- -35 °C
- Boiling point:
- >100 °C (lit.)
- Density
- 1.2 g/mL at 25 °C (lit.)
- vapor density
- 1.3 (vs air)
- vapor pressure
- 613 psi ( 21.1 °C)
- Flash point:
- 10℃ (tag closed test)
- refractive index
- 1.3535
- storage temp.
- Store at +2°C to +25°C.
- solubility
- H2O: soluble
- form
- liquid
- pka
- -7(at 25℃)
- color
- Light Yellow
- Specific Gravity
- 1.19
- PH
- 3.01(1 mM solution);2.04(10 mM solution);1.08(100 mM solution);
- Odor
- Sharp, irritating odor detectable at 0.25 to 10 ppm
- Water Solubility
- miscible
- Sensitive
- Air & Light Sensitive
- Merck
- 14,4780
- Exposure limits
- Ceiling limit 5 ppm (~ 7 mg/m3).
- Dielectric constant
- 4.6(20℃)
- Stability:
- Stable. Incompatible with alkalies, most metals. Avoid contact with water.
- CAS DataBase Reference
- 7647-01-0(CAS DataBase Reference)
- IARC
- 3 (Vol. 54) 1992
- NIST Chemistry Reference
- Hydrogen chloride(7647-01-0)
- EPA Substance Registry System
- Hydrochloric acid (7647-01-0)
Safety Information
- Symbol(GHS)

GHS05
- Signal word
- Warning
- Hazard statements
- H290
- Precautionary statements
- P234-P390
- Hazard Codes
- T,C,F,Xi,F+,Xn
- Safety Statements
- 26-45-36/37/39-9-33-29-16-46-36/37-39
- RIDADR
- UN 2924 3/PG 2
- OEL
- Ceiling: 5 ppm (7 mg/m3)
- WGK Germany
- 2
- RTECS
- MW4025000
- F
- 3
- TSCA
- Yes
- HS Code
- 2806 10 00
- DOT Classification
- 2.3, Hazard Zone C (Gas poisonous by inhalation)
- HazardClass
- 3
- PackingGroup
- I
- Hazardous Substances Data
- 7647-01-0(Hazardous Substances Data)
- Toxicity
- LC50 (30 min) in mice, rats: 2142, 5666 ppm (Darmer)
- IDLA
- 50 ppm
Use
Fire may produce irritating or poisonous gases. Containers may explode in heat of fire. At high temperatures, Hydrochloric acid decomposes into hydrogen and chlorine. The following materials should be avoided: Mercuric sulfate -- violent reaction with gaseous hydrochloric acid at 250F. Sodium -- reacts vigorously with gaseous hydrochloric acid. Acetic anhydride, 2-aminoethanol, ammonium hydroxide, chlorosulfonic acid, ethylene diamine, ethyleneimine, oleum, propiolactone, sodium hydroxide, sulfuric acid, and vinyl acetate -- increase in temperature and pressure when mixed with hydrochloric acid. Calcium phosphide -- energetic reaction with hydrochloric acid. Silver perchlorate and carbon tetrachloride -- when mixed in combination with hydrochloric acid forms a compound that detonates at 105F. Formaldehyde -- when mixed with hydrochloric acid forms a human carcinogen. Material reacts violently with bases and is corrosive with the generation of heat. Reacts with base metals, forming combustible gas (hydrogen). Reacts violently with strong oxidants forming toxic gas (chlorine). Avoid heat; at high temperatures Hydrochloric acid will decompose into hydrogen and chlorine.
According to relevant laws, regulations, policies and the provisions of this website, this website does not display the product information!
The source is "易制毒化學(xué)品". If the information is inaccurate, please contact the platform to modify it.
contact information:info@chemicalbook.com??400-158-660