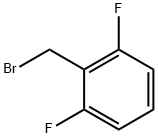
2,6-Difluorobenzyl bromide synthesis
- Product Name:2,6-Difluorobenzyl bromide
- CAS Number:85118-00-9
- Molecular formula:C7H5BrF2
- Molecular Weight:207.02
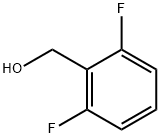
19064-18-7
305 suppliers
$6.00/5g

85118-00-9
332 suppliers
$6.00/1g
Yield:-
Reaction Conditions:
with carbon tetrabromide;triphenylphosphine in diethyl ether at 25; for 2 h;
Steps:
8.B [0182] Description of reaction shown in FIG. 44, Panel B:
In a 50 mL round bottom flask equipped with a magnetic stir bar was charged with a solution of No.136 methyl 2,6-difluorobenzoate (500 mg, 2.90 mmol) in No.42 THF (15 mL) was added dropwise to a solution of No.138 LiAlH4 (200 mg, 5.27 mmol) in THF (15 mL) at 0°C. The resulting solution was allowed to warm to room temperature and stirred for 16 h, and the reaction was quenched by the addition of aq 1 M No.139 KOH (15 mL) dropwise at 0°C. No.38 Et2O (30 mL) was then added, and the mixture stirred for 30 min at room temperature, the layers separated, and the aqueous layer extracted with Et2O (230 mL). The combined organic layers were washed with aq 1 M HCl (30 mL), then brine (30 mL), dried over MgSO4, and concentrated in vacuo to give the No.133 alcohol as a colorless oil, which was used without further purification. A solution of No.133 alcohol (400 mg, 2.77 mmol) in 15 mL of No.38 Et2O containing No.141 triphenylphosphine (728 mg, 2.77 mmol) and No.142 carbon tetrabromide (918 mg, 2.77 mmol) was stirred at 25°C. for 2 h, filtered, and concentrated in vacuo. The crude material was purified by flash chromatography using No.81 hexane:No.82 EtOAc (0-40% EtOAc for 11 cv and ramped to 100% EtOAc for 11-30 cv and then held at 100% EtOAc 30-40 cv) on a 12 g silica column to afford the alkyl bromide. In a 50 mL round bottom flask equipped with a magnetic stir bar was charged with alkyl bromide (450 mg, 2.18 mmol) and No.143 NaN3 (283 mg, 4.36 mmol) in No.144 DMF (3 mL) and was stirred at 50°C. for 24 h. Then the mixture was partitioned between water (50 mL) and Et2O (350 mL). The organic phase was washed with water (210 mL), collected, and dried over MgSO4. The solvent was removed under reduced pressure to afford No.145 2-(azidomethyl)-1,3-difluorobenzene as colorless oil. The three steps in the preparation afforded 2-(azidomethyl)-1,3-difluorobenzene in 68% yield (330 mg, 1.95 mmol).
References:
The Board of Regents for Oklahoma State University;Weaver, Jimmie Dean US2020/399196, 2020, A1 Location in patent:Paragraph 0182

443-84-5
223 suppliers
$11.00/1g

85118-00-9
332 suppliers
$6.00/1g
437-81-0
361 suppliers
$9.00/5g

85118-00-9
332 suppliers
$6.00/1g

13671-00-6
166 suppliers
$9.00/5g

85118-00-9
332 suppliers
$6.00/1g
13648-23-2
1 suppliers
inquiry

85118-00-9
332 suppliers
$6.00/1g