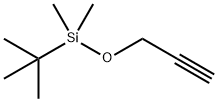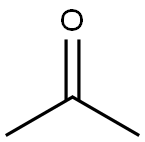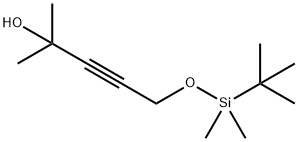
5-{[tert-butyl(dimethyl)silyl]oxy}-2-methylpent-3-yn-2-ol synthesis
- Product Name:5-{[tert-butyl(dimethyl)silyl]oxy}-2-methylpent-3-yn-2-ol
- CAS Number:259880-64-3
- Molecular formula:C12H24O2Si
- Molecular Weight:228.4
Yield:259880-64-3 100%
Reaction Conditions:
Stage #1: tert-Butyldimethyl(prop-2-ynyloxy)silanewith n-butyllithium in tetrahydrofuran;hexane at -78; for 0.5 h;
Stage #2: acetone in tetrahydrofuran;hexane at -78 - 20; for 1 h;
Steps:
25.1 Step 1: 5-{[rt-Butyl(dimethyl)silyl]oxy}-2-methylpent-3-yn-2-ol lnt-49
Step 1: 5-{[rt-Butyl(dimethyl)silyl]oxy}-2-methylpent-3-yn-2-ol lnt-49 teri-Butyldimethyl(2-propynyloxy)silane (2.4 mL, 12 mmol) was dissolved in THF (40 mL) then it was cooled to -78 °C. n-Butyllithium (2.50 M in hexanes; 5.2 mL, 12.9 mmol) was added and the resulting solution allowed to stir for 30 min at -78 °C. Acetone (1.0 mL, 14 mmol) was then added to the reaction, which was then allowed to stir at -78 °C for 30 min, followed by stirring at rt for 30 min. The reaction was then quenched by the addition of saturated aq solution of NH4CI. The mixture was extracted with ether (2x). The combined organic layers were dried over MgS04, filtered, and concentrated to provide 5-{[tert-butyl(dimethyl)silyl]oxy}-2-methylpent-3-yn-2-ol (2.7g, 100%). 'H NMR iCDCls) δ 4.25 (s, 2H), 3.60 (m, 1H), 1.30 (s, 6H), 0.80 (s, 9H), 0.0 (s, 6H).
References:
WO2015/2994,2015,A2 Location in patent:Paragraph 00213
18162-48-6
695 suppliers
$9.00/5g
![5-{[tert-butyl(dimethyl)silyl]oxy}-2-methylpent-3-yn-2-ol](/CAS/20180703/GIF/259880-64-3.gif)
259880-64-3
11 suppliers
inquiry