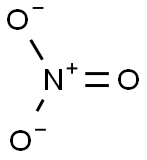NITRATE
- CAS No.
- 14797-55-8
- Chemical Name:
- NITRATE
- Synonyms
- NO3;NITRATE;SEKKEN-K;NITRATE ION;NANO2 99% MIN;SODIUM NITRAT;NITRATE STANDARD;NITROGEN STANDARD;NITRATE IC STANDARD;NITRATE ION STANDARD
- CBNumber:
- CB9748190
- Molecular Formula:
- NO3-
Lewis structure
- Molecular Weight:
- 62
- MDL Number:
- MFCD00144920
- MOL File:
- 14797-55-8.mol
- MSDS File:
- SDS
| storage temp. | 2-8°C |
|---|---|
| form | Liquid |
| color | Clear colorless |
| Water Solubility | Miscible with water. |
| EPA Primary Drinking Water Standard | MCL:10,MCLG:10 |
| FDA 21 CFR | 165.110 |
| EWG's Food Scores | 1 |
| FDA UNII | T93E9Y2844 |
| EPA Substance Registry System | Nitrate (14797-55-8) |
NITRATE price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Alfa Aesar | 035549 | Nitrate, Ion chromatography standard solution, Specpure?, NO3? 1000μg/ml | 14797-55-8 | 100ml | $129 | 2024-03-01 | Buy |
| Alfa Aesar | 035549 | Nitrate, Ion chromatography standard solution, Specpure?, NO3? 1000μg/ml | 14797-55-8 | 500ml | $235 | 2024-03-01 | Buy |
| Alfa Aesar | 023282 | Nitrate Quant?TestStrips | 14797-55-8 | 1kit | $195 | 2023-06-20 | Buy |
| American Custom Chemicals Corporation | ING0008385 | NITRATE 95.00% | 14797-55-8 | 5MG | $505.91 | 2021-12-16 | Buy |
NITRATE Chemical Properties,Uses,Production
Description
Nitrate is commonly found in drinking water sources especially in agricultural areas where nitrogen fertilizer is used, and where unregulated shallow private wells are more at the risk of contamination. The World Health Organization (WHO) guideline of 50 ppm and the US maximum contaminant level (MCL) of 45 ppm for nitrate in drinking water have been established for protecting infants from methemoglobinemia, commonly known as blue baby syndrome. The health protective value continues to be a subject of public health interest for many years, with varying opinion on whether it is too high or too low. Evaluation of nitrate will need to include consideration of nitrite because both are closely related in the nitrogen cycle in the environment and the body, and nitrite plays a major role in inducing toxicity after its formation from nitrate. More recently, reports of nitrate in drinking water, especially at levels higher than 50 ppm, have been associated with other health effects other than methemoglobinemia. This toxicological review provides an update on the health effects of nitrate with a focus on methemoglobinemia, reproductive and developmental effects, potential carcinogenicity, and especially endocrine/thyroid effects.
Chemical Properties
A colorless liquid.
Uses
In the treatment of angina pectoris; in the manufacture of inorganic and organic nitrates and nitro compounds for fertilizers, dye intermediates, explosives, and many different organic chemicals.
Uses
Nitrate is used in fertilizers; in the manufacture of nitrites, nitrous oxide, explosives, pyrotechnics, matches, freezing mixtures, and special cements; as a coloring agent and preserving additive in food; for coagulation of latexes; in the nuclear industry; and for odor (sulfide) and corrosion control in aqueous systems.
Uses
Nitrate is the salt of nitric acid. it is used in meat curing to develop and stabilize the pink color associated with cured meat. by itself, it is not effective in producing the curing reaction until it is chemi- cally reduced to nitrite. it has an effect on flavor and also functions as an antioxidant. it is available as sodium and potassium nitrate, with the sodium form being more common.
Definition
A salt or ester of nitric acid.
Definition
ChEBI: A nitrogen oxoanion formed by loss of a proton from nitric acid. Principal species present at pH 7.3.
Definition
nitrate: A salt or ester of nitric acid.
General Description
Crystalline solids. Salts of nitrate, such as ammonium nitrate, potassium nitrate, and sodium nitrate.
Air & Water Reactions
Most are water soluble.
Reactivity Profile
Mixtures of metal/nonmetal nitrates with alkyl esters may explode, owing to the formation of alkyl nitrates; mixtures a nitrate with phosphorus, tin (II) chloride, or other reducing agents may react explosively [Bretherick 1979. p. 108-109].
Hazard
Moderately toxic.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Environmental Fate
Nitrate (NO3 -), a product of nitrogen oxidation, is a naturally occurring ion in the environment and integrated into complex organic molecules such as proteins and enzymes required by living systems. Nitrate is a more stable form of oxidized nitrogen than nitrite; however, it can be reduced by microbial action to nitrite, which, in turn, can be reduced to various compounds or oxidized to nitrate by chemical and biological processes. Nitrates occur naturally in soil from microbial oxidation of ammonia derived from organic nitrogenous materials such as plant proteins, animals, and animal excreta. Other source contributions are wastewater, septic tank runoffs, airborne nitrogen compounds emitted by industry and automobiles, nitrogen fertilizer, and manure from animal feeding. Nitrate in groundwater is generally found below 10 ppm, with higher levels in areas of high agricultural activities.
Toxicity evaluation
The acute oral LD50 values for sodium nitrate range from 2480
to 9000 mg kg-1 in rats, mice, and rabbits. Acute, subchronic,
and chronic animal toxicity studies showed low toxicity for
nitrate as sodium or potassium nitrate. A long-term study
showed a slight depression in growth rate. Nitrite, but not
nitrate, is capable of inducing methemoglobinemia (see
Nitrites, for more details).
Nitrate has been reported to be associated with thyroid
effects in experimental animals and humans. Possible mode of
action includes inhibition of iodine uptake to thyroid, serum
T3 and T4 changes, and tissue T3 changes. However, there is
a lack of knowledge on the differences in the mode of action to
permit animal-to-human extrapolation. While the data indicate
humans and rats exhibit similar dose–response relationships
in acute inhibition of thyroidal iodide uptake, they show
differences in thyroid hormone response following iodide
uptake inhibition. Comparative data are needed for serum and
brain tissue levels of thyroid hormones and characterization of
the dose–response relationship between changes of thyroid
hormone levels and adverse effects.
Early experimental and field studies in mammals have
found inorganic nitrate to be goitrogenic. The effects were
observed in rats following oral and parenteral administration
of potassium and sodium nitrate, whereas antithyroid effects
were also reported in sheep and pigs administered potassium
nitrate. Nitrate exposure through diet or drinking water caused
functional and histological changes to the thyroid gland in rats
and pigs. More recent investigations between 2000 and 2010
reported changes in thyroid and thyroid activity following
exposure to nitrate. In these more recent studies, nitrate exposure
has consistently resulted in increases in thyroid weight
and/or changes to the follicle cell; however, the reported
thyroidal hormone changes have not been as consistent. The
studies reported increased thyroid weights with a decrease in
thyroid hormones (i.e., T3 and T4) and/or decrease in thyroid
stimulating hormone. However, not all the results are consistent
with the expected outcome of a sodium–iodide symporter
(NIS) inhibitor, which can be seen as supplementation of
iodine in the diet that did not result in thyroid changes.
Overall, the data support that nitrate impairs thyroid function
involving the hypothalamic–pituitary–adrenal axis.
NITRATE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 3010 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | laboratory@coreychem.com | China | 30239 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 7724 | 58 |
| Shanghai Likang New Materials Co., Limited | +86-16631818819 +86-17736933208 | 3684455296@qq.com | China | 9300 | 58 |
| Beijing Yanjing Pharmaceutical Co., Ltd | +86 010-65767987 65763930 | China | 23 | 47 | |
| VWR(Shanghai) Co., Ltd | 400-821-8006 | info_china@vwr.com | China | 3040 | 75 |
| Shanghai QianYan Bio-technology Co., Ltd | 02781293128 | orders@biochemsafebuy.com | China | 9923 | 55 |
| Chengdu Dianchun Technology Co., Ltd | 400-1166-196 18502815961 | cdhxsj@163.com | China | 14603 | 60 |
Related articles
- Uses of Nitrate and Lewis structure
- The Lewis structure of the nitrite ion is made up of a central atom, nitrogen (N), and three oxygen atoms (O), the outer atoms....
- Nov 16,2023