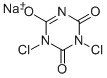
Sodium dichloroisocyanurate
- CAS No.
- 2893-78-9
- Chemical Name:
- Sodium dichloroisocyanurate
- Synonyms
- sdic;nadcc;TROCLOSENE SODIUM;DICHLOROISOCYANURIC ACID SODIUM SALT;DCCNA;BasolanDC(BASF);Sodium troclosene;SodiuM dichloroisocyanurat;acl60;oci56
- CBNumber:
- CB9181452
- Molecular Formula:
- C3Cl2N3NaO3
- Molecular Weight:
- 219.95
- MDL Number:
- MFCD00006036
- MOL File:
- 2893-78-9.mol
- MSDS File:
- SDS
| Melting point | 225°C |
|---|---|
| Density | 1 g/cm3 |
| vapor pressure | 0.006Pa at 20℃ |
| storage temp. | 0-6°C |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly, Heated, Sonicated), Water |
| form | powder to lump |
| color | White to Almost white |
| Water Solubility | 30g/100ml (25 ºC) |
| Sensitive | Moisture Sensitive |
| BRN | 4056155 |
| Stability | Stable. Oxidizing agent - contact with combustible material may lead to fire. Incompatible with strong bases, strong oxidizing agents. Reacts readily with many nitrogen-containing compounds to form explosive nitrogen triiodide. Moisture-sensitive. |
| InChI | InChI=1S/C3HCl2N3O3.Na/c4-7-1(9)6-2(10)8(5)3(7)11;/h(H,6,9,10);/q;+1/p-1 |
| InChIKey | MSFGZHUJTJBYFA-UHFFFAOYSA-M |
| SMILES | C1(=O)N(Cl)C(=NC(=O)N1Cl)[O-].[Na+] |
| Indirect Additives used in Food Contact Substances | SODIUM DICHLOROISOCYANURATE |
| EWG's Food Scores | 1 |
| FDA UNII | 07M9U9U0LK |
| EPA Substance Registry System | Sodium dichloro-s-triazinetrione (2893-78-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS03,GHS05,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H272-H302-H314-H335-H410 | |||||||||
| Precautionary statements | P210-P260-P273-P280-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | O,Xn,N,E | |||||||||
| Risk Statements | 8-22-31-36/37-50/53-2 | |||||||||
| Safety Statements | 8-26-41-60-61-45 | |||||||||
| RIDADR | UN 2465 5.1/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | XZ1900000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29336929 | |||||||||
| Hazardous Substances Data | 2893-78-9(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
Sodium dichloroisocyanurate price More Price(27)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 218928 | Sodium dichloroisocyanurate 96% | 2893-78-9 | 25g | $24.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 218928 | Sodium dichloroisocyanurate 96% | 2893-78-9 | 500g | $53.2 | 2024-03-01 | Buy |
| TCI Chemical | D1003 | Sodium Dichloroisocyanurate >97.0%(T) | 2893-78-9 | 25g | $21 | 2024-03-01 | Buy |
| TCI Chemical | D1003 | Sodium Dichloroisocyanurate >97.0%(T) | 2893-78-9 | 500g | $42 | 2024-03-01 | Buy |
| Alfa Aesar | B23504 | Dichloroisocyanuric acid sodium salt, 97% (dry wt.), water <3% | 2893-78-9 | 25g | $29.4 | 2024-03-01 | Buy |
Sodium dichloroisocyanurate Chemical Properties,Uses,Production
Description
Sodium dichloroisocyanurate(NaDCC) is the sodium salt of a chlorinated
hydroxytriazine and is used as a source of free available chlorine, in
the form of hypochlorous acid, for the disinfection of water. It is
widely used as a stable source of chlorine for the disinfection of
swimming pools and in the food industry. It is also used as a means of
disinfecting drinking-water, primarily in emergencies, when it provides
an easy-to-use source of free chlorine, and, more recently, as the form
of chlorine for household point-of-use water treatment.
Physicochemical properties
Sodium dichloroisocyanurate is white crystalline powder and has a strong smell of chlorine gas, containing available chlorine 60% to 64.5%. It has a stable property, and when stored in high temperature and humid areas, the reduction of available chlorine is only about 1%. It is easily dissolved in water and the solubility is 25%(25℃). The solution is weak acid and PH in 1% of the aqueous solution is 5.8 to 6.0. PH has little change while the concentration of the solution increased. When dissolved in water, sodium dichloroisocyanurate will generate hypochlorous, of which the hydrolysis constant is 1×10-4 higher than that of chloramines-T. The stability of the aqueous solution is poor. Under the ultraviolet light, the loss of the active chlorine would be accelerated. Sodium dichloroisocyanurate solution with low concentration can quickly kill all kinds of vegetative forms of bacteria, fungi and virus ,especially for hepatitis virus. The solution has several characteristics such as with high chlorine content, strong sterilization effect, simple process and low-cost. In addition, sodium dichloroisocyanurate is of low toxicity, and its sterilization effect is better than that of bleaching powder and chloramines-T. Mixing metal reducing agents or acid synergist with potassium permanganate and sodium dichloroisocyanurate powder can produce chlorine smoke fumigant or acid chloride smoke fumigant. Once lighted, such smoke fumigant will give off gas with strong sterilization effect.
Chlorine-containing disinfectants
Sodium dichloroisocyanurate, bleaching powder, dibasic tricalcium hypochlorite and sodium hypochlorite are several chlorine-containing disinfectants which are generally used in China. The disinfection capability of chlorine-containing disinfectant is mainly determined by the content of the available chlorine it contained. The higher the content of available chlorine is, the stronger the ability of disinfection is. When dissolved in water, chlorine-containing disinfectants form hypochlorous which has sterilization effect. Hypochlorous produced by bleaching power or dibasic tricalcium hypochlorite in the solution is associated with the PH of the solution. The lower PH in the solution is, the more hypochlorous it will produce. Due to a higher hydrolysis constant, the sterilization capability of sodium dichloroisocyanurate is stronger than most other chloramine disinfectants. Compared with hypochlorite disinfectants, the sterilization capability of sodium dichloroisocyanurate is weaker in low concentration of solution; while in high concentration of the solution, the solution can keep weak acidity. Therefore, the sterilization effect of sodium dichloroisocyanurate sometimes can be better than that of hypochlorite disinfectants. Chlorine-containing disinfectants mentioned above have good broad-spectrum germicidal efficacy and killing effects on vegetative form of bacteria, virus, fungal spore and bacterial spore.
sodium dichloroisocyanurate is one of the chlorinated isocyanuric acid disinfectants. Congeneric products also include chloroisobromine acid, trichloroisocyanuric acid, dichlord isocyanurice acid, potassium dichloroisocyanurate and so on. This product and other chlorinated isocyanuric acid disinfectants have a wide bactericidal spectrum and strong sterilization effect on vegetative form of bacteria, virus, fungal spore and bacterial spore. They also have strong killing effect on various bacterial(eg. escherichia coli, staphylococcus aureus ), virus(eg. hepatitis virus), bacterial spores(eg. spores of bacillus subtilis var niger) and fungus. In the effective concentration of the solution, this product can kill 99.9% escherichia in 30 minutes, all bacteria spores in five to thirty minutes and all hepatitis virus in five minutes. Besides, this product also have the function to destroy surface antigen of type B hepatitis virus. Sodium dichloroisocyanurate has a better killing effect in the acidic conditions. The lower PH is, the better effect it has. This product has a good stability of chemical property and exists no carcinogenic potential. This product can be used for disinfection in water, processing equipment in food factory, tableware, food, vehicles, animal buildings, magnanerie, fishpond and utensil and so on.
The above information is edited by the Chemicalbook Bai Linlin.
Uses
Sodium dichloroisocyanurate, the sodium salt of a chlorinated hydroxytriazine, is used as a source of free available chlorine (in the form of hypochlorous acid, HOCl) for disinfecting drinking water.
Sodium dichloroisocyanurate can be manufactured either as the anhydrous salt or as the dihydrate. Anhydrous sodium dichloroisocyanurate contains about 63% free available chlorine. When this compound is added to water, it is rapidly hydrolyzed to release free available chlorine, establishing a complex series of equilibria involving six chlorinated and four non-chlorinated isocyanurates. As free available chlorine is consumed on reacting with organic material in the water, chloroisocyanurates rapidly dissociate and continue to release free chlorine.
The use of sodium dichloroisocyanurate as a source of freely available chlorine is not expected to lead to greater production of by-products than does the use of elemental chlorine.
Chemical Properties
Dichloroisocyanuric acid, sodium salt, is a white crystalline powder. Chlorine odor. Thermally unstable. It is produced as a result of reaction of cyanuric acid with chlorine. Sodium dichloroisocyanurate is a well established disinfectant used for many purposes including wound cleansing, hospital use, sterilizing babies bottles, disinfection of water for human consumption and disinfection of swimming pools.
Characteristics
Sodium dichloroisocyanurate (NaDCC) is a broad-spectrum disinfectant agent:
More stable than bleach (sodium hypochlorite) - consistent strength produced at point of use for cleaning and disinfecting hard surfaces;
Almost neutral in pH - less corrosive on surfaces than liquid bleach;
Biodegradable – safe for the environment.
Uses
Sodium dichloroisocyanurate can be used as a reagent for N-monochlorination and dehydrochlorination of amino esters. It is also a reagent for chlorination to detect ammonium via formation of colored zebra-bands in a detecting tube.
Uses
Sodium Dichloroisocyanurate (SDIC) widely applied for the sterilization of swimming pool and drinking water, or fighting against infectious diseases, or act as disinfectant in raising silkworm, livestock, poultry and fish. Other applications of SDIC are found in wool shrinkage, textile bleaching, and industrial circulating water cleaning. SDIC is normally supplied in powder and granular, tablets are also available on request. Stabilised chlorine granular (dichlor) are used very widely to chlorinate swimming pool water.
Preparation
Sodium dichloroisocyanurate is produced by chlorination of disodium cyanurate [Na2H(NCO)3] using chlorine (CI2) and neutralization with sodium hydroxide (NaOH).
Disodium cyanurate is obtained by action of sodium hydroxide on isocyanuric acid.
General Description
White solid with an odor of bleach-like odor. Mixes with water.
Air & Water Reactions
Water soluble. May vigorously react with water releasing chlorine gas. Material containing less than 39% available chlorine will undergo reactions as described herein, but may take longer to initiate, and the resulting reaction may not be as vigorous [AAR 1992].
Reactivity Profile
Contact with ammonium compounds or hydrated salts can cause a very vigorous reaction. Prolonged exposure to heat /fire may result in the vigorous decomposition of the material with the rupture of its containers, Sodium dichloroisocyanurate will accelerate the burning of combustible materials [AAR 1991]. Chlorine plus alcohols would yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites [NFPA 491 M 1991].
Hazard
Strong oxidizing material, fire risk near organic materials. Toxic by ingestion.
Health Hazard
Dust causes sneezing and coughing, moderate irritation of the eyes, and itchiness and redness of the skin. Ingestion causes burns of mouth and stomach.
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic to humans and animals by ingestion. An experimental teratogen. Experimental reproductive effects. A severe skin and eye irritant. Human systemic effects by ingestion: ulceration or bleeding from stomach. The other main toxic effects were gastrointestinal irritation, salivation, lachrymation, dyspnea, weakness, emaciation, lethargy, diarrhea, coma, and (following very high dosage) death after 1-8 days, with autopsy showing irritation of stomach and gastrointestinal tract, liver dysfunction, and lung congestion. The concentrated material may be a little more toxic, due to greater gastrointestinal irritation. In the dry form, it is not appreciably irritating to dry skin. However, when moist, the concentrated material is irritating to skin, and also may cause severe eye irritation. A powerful oxidizer. Incompatible with combustible materials, ammonium salts, nitrogenous materials. Used to chlorinate swimming pools and in cleaning, bleaching, disinfecting, sanitizing. When heated to decomposition it emits very toxic fumes of Cl-, NOx, and Na2O.
Potential Exposure
Dichloroisocyanuric acid salts, are used in cleaning; making dry bleaches, detergents, sanitizers, and disinfectants; in swimming pool and sewage treatment.
Shipping
UN2465 Dichloroisocyanuric acid, dry or Dichloroisocyanuric acid salts, Hazard Class: 5.1; Labels: 5.1-Oxidizer.
Incompatibilities
A powerful oxidizer. Dust may form explosive mixture with air. Violent reaction with reducing agents; organic matter; easily chlorinated or oxidized materials. Isocyanates are highly flammable and reactive with many compounds, even with themselves. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Reaction with moist air, water or alcohols may form amines and insoluble polyureas and react exothermically, releasing toxic, corrosive or flammable gases, including carbon dioxide; and, at the same time, may generate a violent release of heat increasing the concentration of fumes in the air. Incompatible with amines, aldehydes, alkali metals, ammonia, carboxylic acids, caprolactum, alkaline materials, glycols, ketones, mercaptans, hydrides, organotin catalysts, phenols, strong acids, strong bases, strong reducing agents such as hydrides, urethanes, ureas. Elevated temperatures or contact with acids, bases, tertiary amines, and acyl-chlorides may cause explosive polymerization. Contact with metals may evolve flammable hydrogen gas. Attacks some plastics, rubber and coatings May accumulate static electrical charges, and may cause ignition of its vapors. Incompatible with ammonium salts, amines forming nitrogen trichloride
Sodium dichloroisocyanurate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Shengyuan Jinlong Import and Export Co. LTD | +8617367750398 | info@hebeisyjl.com | China | 1838 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 | lisa@kingfinertech.com | China | 3010 | 58 |
| Hebei Mojin Biotechnology Co.,Ltd | +86-15028179902 | angelia@hbmojin.com | China | 1176 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2961 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8810 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5857 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | sales@myuchem.com | China | 7629 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5889 | 58 |
| Hebei Dangtong Import and export Co LTD | +86-13910575315 +86-13910575315 | admin@hbdangtong.com | China | 1000 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8617732866630 | bess@weibangbio.com | China | 18151 | 58 |
Related articles
- Application and toxicity of sodium dichloroisocyanurate
- Sodium dichloroisocyanurate(NaDCC) is the sodium salt of a chlorinated hydroxytriazine and is used as a source of free availab....
- Aug 15,2022
View Lastest Price from Sodium dichloroisocyanurate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-12-22 | Sodium dichloroisocyanurate
2893-78-9
|
US $999.00-800.00 / ton | 1ton | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-12-20 | Sodium dichloroisocyanurate
2893-78-9
|
US $3.00 / KG | 1KG | ≥60%; ≥56% | 2000kg | Jinan Finer Chemical Co., Ltd | |
 |
2024-12-17 | Sodium Dichloroisocyanurate
2893-78-9
|
US $10.00 / kg | 1kg | 99% | 10000 | Hebei Miaoyin Technology Co.,Ltd |
-

- Sodium dichloroisocyanurate
2893-78-9
- US $999.00-800.00 / ton
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Sodium dichloroisocyanurate
2893-78-9
- US $3.00 / KG
- ≥60%; ≥56%
- Jinan Finer Chemical Co., Ltd
-

- Sodium Dichloroisocyanurate
2893-78-9
- US $10.00 / kg
- 99%
- Hebei Miaoyin Technology Co.,Ltd