410528-02-8
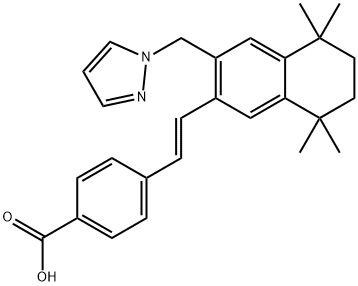 410528-02-8 結(jié)構(gòu)式
410528-02-8 結(jié)構(gòu)式
基本信息
4-[(1E)-2-[5,6,7,8-四氫-5,5,8,8-四甲基-3-(1H-吡唑-1-基甲基)-2-萘基]乙烯基]苯甲酸
Ro 3300074
palovarotene
Unii-28K6I5m16g
R 667
RO 3300074
Palovarotene(R 667)
R667
R 667
R667
RO-3300074
RO 3300074
RO3300074
4-[(E)-2-[5,5,8,8-tetramethyl-3-(pyrazol-1-ylmethyl)-6,7-dihydronaphthalen-2-yl]ethenyl]benzoic acid
(E)-4-(2-(3-((1H-pyrazol-1-yl)Methyl)-5,5,8,8-tetraMethyl-5,6,7,8-tetrahydronaphthalen-2-yl)vinyl)benzoic acid
4-[(1E)-2-[5,6,7,8-Tetrahydro-5,5,8,8-tetramethyl-3-(1H-pyrazol-1-ylmethyl)-2-naphthalenyl]ethenyl]benzoic acid
物理化學(xué)性質(zhì)
常見問題列表
RAR-γ
Palovarotene suppresses post-traumatic chondrogenesis and osteogenesis and mitigated trauma-induced ectopic bone formation. Palovarotene inhibits subcutaneous and intramuscular heterotopic ossification (HO) in mice. Palovarotene is given orally for 14 days at 1 mg/kg/day starting on post-operative day (POD) 1 or POD-5, and HO amount, wound dehiscence and related processes are monitored for up to 84 days post injury. Compared to vehicle-control animals, Palovarotene significantly decreases HO by 50 to 60% regardless of when the treatment started and if infection is present. Starting from day 1 of injury, half of the Acvr1 cR206H/+ mice are treated with Palovarotene by daily gavage for 14 days and the other half received vehicle as control. Analysis by mCT and 3D image reconstruction at day 14 shows that large HO tissue masses have formed in the targeted leg of Acvr1 cR206H/+ mutant mice receiving vehicle, but HO formation is greatly diminished in Palovarotene-treated companions by more than 80% based on bone volume/total volume quantification.
