1222102-29-5
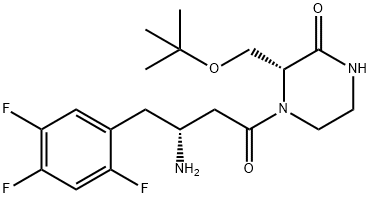 1222102-29-5 結(jié)構(gòu)式
1222102-29-5 結(jié)構(gòu)式
基本信息
中文別名
依格列汀 英文別名
Evogliptin2-Piperazinone, 4-[(3R)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-3-[(1,1-dimethylethoxy)methyl]-, (3R)-
物理化學(xué)性質(zhì)
沸點(diǎn)571.5±50.0 °C(Predicted)
密度1.234±0.06 g/cm3(Predicted)
酸度系數(shù)(pKa)14.09±0.40(Predicted)
應(yīng)用領(lǐng)域
用途一
Developed by Dong-A ST,
evogliptin was approved in 2015 in the Republic of Korea for
blood glucose control in patients with diabetes mellitus type 2
(type 2 DM). Evogliptin is an orally bioavailable dipeptidyl
peptidase IV (DPP-4) inhibitor, which acts to prevent insulin
secretion following meals. Dong-A ST arranged licensing
agreements with Geropharm and Eurofarma Laboratórios for
the sale of evogliptin in various countries in eastern Europe and
Brazil, respectively, pending future approvals. While a
manufacturing route has not been disclosed to date, the most
scalable published route is described below.