120241-31-8
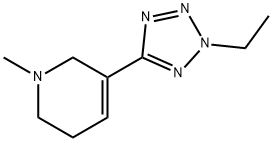 120241-31-8 結(jié)構(gòu)式
120241-31-8 結(jié)構(gòu)式
基本信息
ALVAMELINE
3-(2-Ethyl-2H-tetrazol-5-yl)-1,2,5,6-tetrahydro-1-methylpyridine
Pyridine, 3-(2-ethyl-2H-tetrazol-5-yl)-1,2,5,6-tetrahydro-1-methyl-
2-Ethyl-5-(1-methyl-1,2,5,6-tetrahydro-3-pyridyl)-2H-tetrazole tartrate
5-(2-Ethyl-2H-tetrazol-5-yl)-1,2,3,6-tetrahydro-1-methylpyridine tartrate
3-(2-Ethyl-2H-tetrazol-5-yl)-1,2,5,6-tetrahydro-1-methyl-pyridine tartrate
物理化學(xué)性質(zhì)
常見問題列表
Alvameline is metabolized by human liver microsomes to Lu 31-126 mainly by CYP2D6; to Lu 29-297 and Lu 25-077 mainly by CYP1A2, CYP2A6, CYP2C19, and CYP3A4; and to Lu 32-181 by CYP1A2 and possibly by CYP2C19. One metabolite, Lu 32-181, could be reduced back to alvameline, a reaction not inhibited by the applied cytochrome P-450 inhibitors.
Alvameline competitively and effectively antagonizes carbachol-induced contractions and contractions induced by electrical field stimulation in human detrusor muscle. Alvameline produces a concentration-dependent rightward shift of the concentration-response curves for carbachol in both human and pig detrusor, the pK b values being 6.2 and 5.8. Contractions induced by electrical field stimulation in human detrusor are almost completely inhibited by 100 μM alvameline. In contrast, electrical field stimulation-induced contractions in pig detrusor are less sensitive to alvameline, resulting in a final inhibition of 32% with the highest concentration used (100 μM). Alvameline has been shown to improve cognitive function following traumatic brain injury in rats. Alvameline treated rats causes a 13% and 5% decrease in the medial septal nucleus, a 48 and 23% decrease in the vertical limb nucleus of the diagonal band, and a 51 and 28% decrease in the nucleus basalis magnocellularis, respectively.