115551-26-3
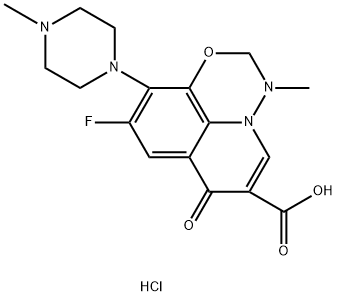 115551-26-3 結(jié)構(gòu)式
115551-26-3 結(jié)構(gòu)式
基本信息
9-fluoro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-2,3-dihydro-7H-pyrido[3,2,1-ij]-1,3,4-benzoxadiazine-6-carboxylic acid hydrochloride
常見(jiàn)問(wèn)題列表
Administration of Marbofloxacin at 6 mg/kg once daily for 7 days in a Staphylococcus aureus infection in tissue cages in ponies is not effective for the elimination of S. aureus infections from secluded sites [2]. The pharmacokinetic properties of marbofloxacin were investigated in 6 horses after i.v., subcutaneous and oral administration of a single dose of 2 mg/kg bwt and the minimal inhibitory concentrations (MIC) assessed for bacteria isolated from equine infectious pathologies. The clearance of marbofloxacin was mean +/- s.d. 0.25 +/- 0.05 l/kg/h and the terminal half-life 756 +/- 1.99 h. The marbofloxacin absolute bioavailabilities after subcutaneous and oral administration were 98 +/- 11% and 62 +/- 8%, respectively. Considering the breakpoint values of efficacy indices for fluoroquinolones, a marbofloxacin dosage regimen of 2 mg/kg bwt/24 h by i.v., subcutaneous or oral routes was more appropriate for enterobacteriaceae than for S. aureus [3].