1044589-82-3
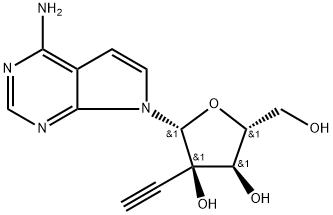 1044589-82-3 結(jié)構(gòu)式
1044589-82-3 結(jié)構(gòu)式
基本信息
7-Deaza-2'-C-ethynyladenosine
7-Deaza-2'-C-acetylene-adenosine
7-(2-C-Ethynyl-β-D-ribofuranosyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine
7H-Pyrrolo[2,3-d]pyrimidin-4-amine, 7-(2-C-ethynyl-β-D-ribofuranosyl)-
(2R,3R,4R,5R)-2-(4-Amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-3-ethynyl-5-(hydroxymethyl)tetrahydrofuran-3,4-diol
物理化學(xué)性質(zhì)
常見問題列表
EC50: 0.64 μM ( DENV-2 )
NITD008 potently inhibits other, including Dengue virus (DENV), West Nile virus, yellow fever virus, and Poissan virus. NITD008 inhibits DENV-2 in a dose-responsive manner, with an EC 50 value of 0.64 μM; treatment with 9 μM compound reduces viral titer by >104-fold. NITD008 also inhibits a luciferase-reporting replicon of hepatitis C virus (HCV, genotype 1b), a member from the genus Hepacivirus, with an EC 50 value of 0.11 μM.
NITD008 is orally bioavailable and has good pharmacokinetic properties. NITD008 exhibits the best pharmacokinetic parameters when formulated using 6 N of HCl (1.5 equimolar amount), 1 N of NaOH (pH adjusted to 3.5), and 100 mM citrate buffer (pH 3.5). Following i.v. injection, NITD008 has a high volume of distribution (3.71 L/kg) and a low systemic clearance (31.11 mL/min per kg), resulting in a long elimination half-life (t 1/2 =4.99 h). After p.o. dosing, NITD008 is rapidly absorbed (time of peak plasma concentration=0.5 h), with a maximal plasma concentration of 3 μM and bioavailability of 48%. Treatment of the mice immediately after viral infection with 1 mg/kg of NITD008 does not reduce mortality, but treatment with 3 mg/kg partially protects and treatment with ≥10 mg/kg completely protects the infected mice from death. NITD008 can suppress peak viremia, decrease cytokine elevation, and prevent death.