| Identification | Back Directory | [Name]
methyl 2-fluoro-3-nitrobenzoate | [CAS]
946126-94-9 | [Synonyms]
2-fluoro-3-nitrobenzoate
methyl 2-fluoro-3-nitrobenzoate
2-Fluoro-3-(methoxycarbonyl)nitrobenzene
Benzoic acid, 2-fluoro-3-nitro-, Methyl ester | [EINECS(EC#)]
689-721-5 | [Molecular Formula]
C8H6FNO4 | [MDL Number]
MFCD10566813 | [MOL File]
946126-94-9.mol | [Molecular Weight]
199.14 |
| Chemical Properties | Back Directory | [Melting point ]
77- 80° C | [Boiling point ]
310.8±27.0 °C(Predicted) | [density ]
1.388±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Solid | [color ]
Off-White to Pale Yellow |
| Hazard Information | Back Directory | [Uses]
Methyl 2-fluoro-3-nitrobenzoate can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development processes and chemical production processes. | [Synthesis]
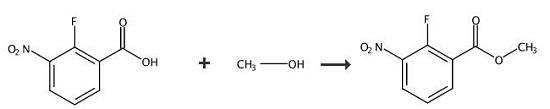 A solution of 2-fluoro-3-nitrobenzoic acid (5 g, 27.0 mmol) in MeOH (50 mL) was treated with concentrated H2SO4 (1.4 mL, 27.0 mmol) and the resulting reaction was stirred at 50 °C for 16 h. The solvent was removed under reduced pressure and the residue was diluted with EtO Ac. The organic solution was washed with saturated aqueous NaHCO3 solution until the water phase reached neutral pH. The two phases were separated. Organics were washed with water, brine and dried (Na2SO4). The solution was filtered and concentrated to give crude methyl 2-fluoro-3-nitrobenzoate which was used as is in the next step. (5.1 g, 25.2 mmol, 93 %) as a pale yellow solid. A solution of 2-fluoro-3-nitrobenzoic acid (5 g, 27.0 mmol) in MeOH (50 mL) was treated with concentrated H2SO4 (1.4 mL, 27.0 mmol) and the resulting reaction was stirred at 50 °C for 16 h. The solvent was removed under reduced pressure and the residue was diluted with EtO Ac. The organic solution was washed with saturated aqueous NaHCO3 solution until the water phase reached neutral pH. The two phases were separated. Organics were washed with water, brine and dried (Na2SO4). The solution was filtered and concentrated to give crude methyl 2-fluoro-3-nitrobenzoate which was used as is in the next step. (5.1 g, 25.2 mmol, 93 %) as a pale yellow solid. |
|
|