| Identification | Back Directory | [Name]
Fluxametamide | [CAS]
928783-29-3 | [Synonyms]
FLUXAMETAMIDE
4-[5-(3,5-Dichlorophenyl)-4,5-dihydro-5-(trifluoromethyl)-3-isoxazolyl]-N-[(methoxyamino)methylene]-2-methylbenzamide
Benzamide, 4-[5-(3,5-dichlorophenyl)-4,5-dihydro-5-(trifluoromethyl)-3-isoxazolyl]-N-[(methoxyamino)methylene]-2-methyl- | [EINECS(EC#)]
813-746-4 | [Molecular Formula]
C20H16Cl2F3N3O3 | [MOL File]
928783-29-3.mol | [Molecular Weight]
474.26 |
| Chemical Properties | Back Directory | [density ]
1.43±0.1 g/cm3(Predicted) | [storage temp. ]
Store at -20°C | [solubility ]
DMSO : 125 mg/mL (263.57 mM);Water : < 0.1 mg/mL (insoluble) | [form ]
Solid | [pka]
11.29±0.46(Predicted) | [color ]
White to off-white |
| Hazard Information | Back Directory | [Description]
Fluxametamide is a novel isoxazoline insecticide that acts via distinctive antagonism of insect ligand-gated chloride channels, acts as an antagonist of GABA- and glutamate-gated chloride channels(IC50 of 1.95 nM and 225 nM for M. domestica GABACls and GluCls). | [Uses]
Fluxametamide is a novel insecticide belongs to a class of compounds called isoxazolines, which are potent inhibitors of γ-aminobutyric acid (GABA)-, glutamate-, and glycine-gated chloride channels in insects. However, this pesticidal mode of action (MOA) does not seem to be operative in mammals as neurotoxicity was not found in either the acute or subchronic neurotoxicity studies at the limit dose. | [Definition]
ChEBI: 4-[5-(3,5-dichlorophenyl)-5-(trifluoromethyl)-4,5-dihydro-1,2-oxazol-3-yl]-N-[(methoxyamino)methylidene]-2-methylbenzamide is a benzamide obtained by formal condensation of the carboxy group of 4-[5-(3,5-dichlorophenyl)-5-(trifluoromethyl)-4,5-dihydro-1,2-oxazol-3-yl]-2-methylbenzoic acid with the amino group of N-methoxymethanimidamide. It is a member of benzamides, an isoxazoline, a dichlorobenzene, a member of formamidines, an organofluorine compound and an ether. | [Synthesis]
The synthesis route of fluxametamide is shown in Fig. 1. As mentioned earlier, the isoxazoline 9 can be cyclized by reacting the olefin 5 and the chloroaldoxime 8 that are derived from the boronic acid 4 and the aldehyde 6, respectively. CO insertion of 9, followed by the addition of ammonia to carboxylic acid chloride, affords carbamoyl 11.
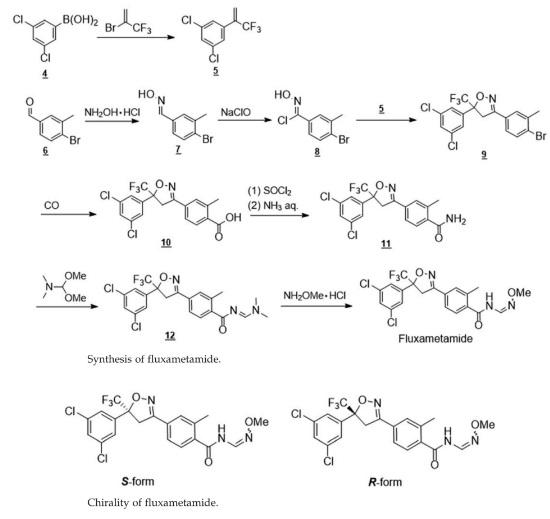
After dimethylamino methylation with N,N-dimethylformamide dimethyl acetal, the dimethylamino group can be converted into the methoxyimino group by using methox- yamine hydrochloride.
The asymmetric center of the isoxazoline ring has an insecticidal activity in its S-form, but fluxametamide was developed as a racemate. | [in vitro]
Fluxametamide is an antagonist of GABA- and glutamate-gated chloride channels, dose-dependently inhibits currents induced by GABA and glutamate in M. domestica GABACls and GluCls, with IC50 values of 1.95 (1.18-3.21) nM and 225 (137-372) nM, respectively, and displays potent antagonistic activity against T. urticae GABACls with an IC50 of 0.219 (0.127-0.381) nM. Fluxametamide inhibits GABA responses in the wild-type L. striatellus GABACls with IC50 values of 1.40 (0.57-3.29) nM; in the A2′N mutant GABACls, the IC50 value is 3.51 (2.17-5.69) nM. Moreover, Fluxametamide scarcely inhibits GABA (EC50)-induced currents in rat GABACls at 10 μM and with no inhibition on glycine (EC50)-induced current in human α1 GlyCls at tested concentrations. |
|
| Company Name: |
Madision Biomedical Co., Ltd. Gold
|
| Tel: |
028-87439248 19150327610 |
| Website: |
http://www.approvedhomemanagement.com/ShowSupplierProductsList30539/0.htm |
|