| Identification | Back Directory | [Name]
Canagliflozin heMihydrate | [CAS]
928672-86-0 | [Synonyms]
Cageline
TA7284 hemihydrate
TA-7284 hemihydrate
Invokana hemihydrate
Canagliflozin hydrate
JNJ28431754 hemihydrate
Cagliflozin heMihydrate
JNJ 28431754 hemihydrate
JNJ-28431754 hemihydrate
Canagliflozin heMihydrate
Canagli ozin Hemi hydrate
Canagliflozin heMihydrates
Canagliflozin hydrate (2:1)
Canagliflozin (API)
D-Glucitol, 1,5-anhydro-1-C-[3-[[5-(4-fluorophenyl)-2-thienyl]Methyl]-4-Methylphenyl]-, hydrate (2:1)
(1S)-1,5-Anhydro-1-C-[3-[[5-(4-fluorophenyl)-2-thienyl]methyl]-4-methylphenyl]-D-glucitol hydrate (2:1)
bis((2S,3R,4R,5S,6R)-2-(3-{[5-(4-fluorophenyl)thio phen-2-yl]methyl}-4-methylphenyl)-6-(hydroxymet hyl)oxane-3,4,5-triol) hydrate
JNJ28431754 HEMIHYDRATE; JNJ-28431754 HEMIHYDRATE; JNJ 28431754 HEMIHYDRATE; TA-7284 HEMIHYDRATE; TA7284 HEMIHYDRATE; INVOKANA HEMIHYDRATE
(2S,3R,4R,5S,6R)-2-(3-((5-(4-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol Hemihydrate | [EINECS(EC#)]
202-303-5 | [Molecular Formula]
2(C24H25FO5S).H2O | [MDL Number]
MFCD28975933 | [MOL File]
928672-86-0.mol | [Molecular Weight]
462.53 |
| Chemical Properties | Back Directory | [Melting point ]
94-96°C | [storage temp. ]
Hygroscopic, Refrigerator, under inert atmosphere | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [color ]
White to Off-White | [Stability:]
Hygroscopic |
| Hazard Information | Back Directory | [Description]
Canagliflozin, an orally active and selective sodium–glucose
cotransporter 2 (SGLT2) inhibitor, was co-developed by
Mitsubishi Tanabe Pharma and Johnson & Johnson (J&J) for the
treatment of type 2 diabetes mellitus (T2DM) and obesity. The
drug was approved in March by the U.S. FDA and launched in
April 2013 in the U.S. SGLT2 is involved in the glucose re-absorption
pathway in the kidney, and its inhibition increases urinary
glucose excretion, and reduces plasma glucose and HbA1c levels.
In addition, canagliflozin is safe in combination with other commonly
used antidiabetic agents and has a significant effect on body
weight reduction. A recently published process patent from
ScinoPharm Taiwan describes the synthesis of canagliflozin. | [Uses]
Canagliflozin Hemihydrate is a derivative of Canagliflozin (C175190), which is a sodium/glucose cotransporter 2 (SGLT2) inhibitor. Canagliflozin has been shown to dose dependently reduce calculated renal threshold for glucose excretion and increase urinary glucose excretion. Canagliflozin is a candidate for the treatment of type 2 diabetes and obesity. | [Definition]
ChEBI: Canagliflozin hydrate is a hydrate that is the hemihydrate form of canagliflozin. Used for treatment of type II diabetes via inhibition of sodium-glucose transport protein subtype 2. It has a role as a hypoglycemic agent and a sodium-glucose transport protein subtype 2 inhibitor. It contains a canagliflozin. | [Synthesis]
Synthesis of the aglycone region of canagliflozin was described
in a separate patent by first condensing commercially available 5-
bromo-2-methylbenzoyl chloride (14) and 2-(4-fluorophenyl)-
thiophene (15) under Friedel¨CCrafts acylation conditions to give
ketone 16 in 69% yield as a crystalline solid. Ketone 16 was then
reduced with triethylsilyl hydride in the presence of BF3�Et2O at
low temperature to give aglycone bromide 17 in 70% yield. The
precursor for the glycoside moiety, commercially available glycoside
triol 18, was selectively treated with t-butyldiphenylsilyl
chloride (TBDPSCl) in THF in the presence of imidazole to give
the bis-silyl ether 19 in 81% yield. Next, a unique, stereospecific
b-C-arylglucosidation was developed to secure the union of the
aglyone- and glycoside-containing portions of canagliflozin.
Bromide 17 was subjected to magnesium powder under standard
Grignard conditions prior to treatment with AlCl3 in THF in situ.
This resulting mixture was then exposed to a solution of compound
19 in PhOMe which had been pre-treated with n-BuLi, and the entire mixture was then warmed to 150 ?? for 5 h to ultimately
give the b-anomer 20 in 56% yield. Finally, removal of the silyl
groups within 20 with tetrabutyl ammonium fluoride (TBAF) in
THF delivered canagliflozin hydrate (III) in 73% yield.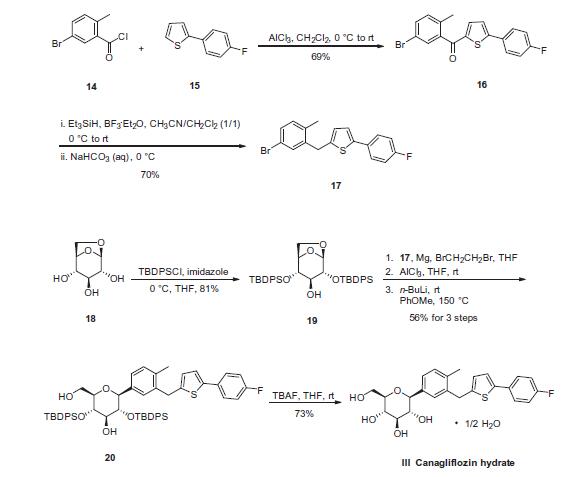
| [storage]
Store at -20°C |
|
|