| Identification | Back Directory | [Name]
Dextran | [CAS]
9004-54-0 | [Synonyms]
LMD
LVD
LMWD
PL 1S
Hyscon
LU 122
Hyskon
Onkotin
DEX 500
Gentran
Hemodex
Macrose
DEXTRAN
MUCROSE
Plavolex
Intradex
Gentran3
dextran2
dextran5
dextrans
Macrodex
Infucoll
Intrader
DextranuM
Dextranen
Eudextran
Detrax 40
INDOLAXIN
Dextraven
Ex-pandex
dextran11
DEXTRAN 1
DEXTRAN 4
DEXTRAN 8
DEXTRAN 60
DEXTRAN-70
DEXTRAN 35
DEXTRAN-40
DEXTRAN 10
polyglusol
DEXTRAN 15
Dextran T3
Dextran T4
Dextran T5
Dextran T6
Dextran T7
Dextran T9
Gentran 40
Dextran T50
Dextran T20
Dextran T11
DEXTRAN 150
DEXTRAN FP1
DEXTRAN 100
DEXTRAN 200
DEXTRAN 250
DEXTRAN 500
DEXTRAN T 70
DEXTRAN 2000
rheomacrodex
DEXTRAN FP40
DEXTRAN FP60
DEXTRAN FP70
DEXTEMPO(TM)
DEXTRAN T 40
DEXTRANE 200
DEXTRANE 4-6
ALPHA-GLUCAN
Dextrane2000
Dextran T110
Dextran T100
Dextran T200
Polyglucinum
Oncovertin N
Dextran D-20
Dextran D-40
Dextran D-50
Dextran D-60
Dextran D-70
Dextran T1000
DEXTRAN T 500
DEXTRAN T 250
DEXTRAN 1'000
DEXTRANE 8-12
DEXTRAN 5,000
DEXTRAN 5'000
DEXTRAN 70000
DEXTRAN 6,000
DEXTRAN 75,000
DEXTRAN 80'000
DEXTRAN 50'000
DEXTRAN 12'000
DEXTRAN, 3,000
DEXTRAN 40,000
DEXTRAN 10,000
DEXTRANE 60-90
DEXTRANE 35-50
NATIVE DEXTRAN
Dextran D-500
Longasteril 70
Dextran 20,000
Dextran D-1000
Rheopolyglucine
Dextran10,000Mw
Dextran70,000Mw
Dextran75,000Mw
Dextran40,000Mw
DEXTRAN 150,000
DEXTRAN 150'000
DEXTRAN 200,000
DEXTRAN, 25,000
DEXTRAN 250,000
DEXTRAN 270'000
DEXTRAN 500,000
DEXTRAN 410'000
DEXTRAN 670'000
Glucose polymer
DEXTRANE 400-600
DEXTRANE 100-200
Dextran500,000Mw
Dextran150,000Mw
DextrangradeA,B,C
Dextran 1 (50 mg)
Dextran [Mw 20000]
Dextran 40 (50 mg)
Dextran 70 (50 mg)
Dextran [Mw 70000]
Dextran [Mw 40000]
Dextran, , Mucrose
Dextran [Mw 100000]
Dextran [Mw 150000]
Dextran [Mw 250000]
DEXTRAN,3,000,POWDER
DEXTRAN,5,000,POWDER
DEXTRAN,70,000,POWDER
DEXTRAN,75,000,POWDER
DEXTRAN,40,000,POWDER
lowmoleculardextran-l
DEXTRAN 4,000-6,000
Dextran T-10 (200 mg)
DEXTRAN STANDARDS KIT
Dextran 40 Calibration
Dextran, 4,000, Powder
DEXTRAN 8,000-12,000
DEXTRAN,150,000,POWDER
DEXTRAN,250,000,POWDER
DEXTRAN,500,000,POWDER
DEXTRAN MOL. WT. 70,000
DEXTRAN 60,000-90,000
DEXTRAN 35,000-50,000
DEXTRAN 32,000-48,000
DEXTRAN 15,000-20,000
DEXTRAN MOL. WT. 500,000
Dextran enzymatic synth.
Dextran [Mw.=ca. 500000]
Dextran MW 40,000 ± 3,000
DEXTRAN 200,000-300,000
DEXTRAN MOL. WT. 2,000,000
DEXTRAN STANDARD 4'900'000
Dextran Vo Marker (100 mg)
DEXTRAN FP40 RESEARCH GRADE
Dextran 40 (Mw.=ca. 40,000)
Dextran 70 (Mw.=ca. 70,000)
Dextran, average M.W. 5,000
Dextran75,000Mw,PyrogenFree
POLY[(1,6)-ALPHA-D-GLUCOSE]
Dextran, average M.W. 10,000
Dextran, average M.W. 70,000
Dextran, average M.W. 20,000
Dextran FP 70 research grade
Dextran, 40,000, Powder, USP
Dextran ,0% [Mw.=ca. 500000]
Dextran, average M.W. 500,000
Dextran, average M.W. 100,000
Dextran, average M.W. 150,000
Dextran (Technical Grade~ 6K)
DEXTRAN FROM LEUCONOSTOC SSP.
DextranMw5,000,000-40,000,000
dextran from leuconostoc spp.
Dextran (Technical Grade~ 10K)
Dextran (Technical Grade~ 20K)
Dextran (Technical Grade~ 40K)
Dextran (Technical Grade~ 70K)
Dextran 4 Calibration
Dextran 4 Calibration (100 mg)
Low molecular weight dextran L
iron dextran complex injection.
Dextran 10 Calibration (100 mg)
Dextran 40 Calibration (100 mg)
Dextran 70 Calibration (100 mg)
Dextran 40 pharmaceutical grade
Dextran 50 pharmaceutical grade
Dextran 70 pharmaceutical grade
Dextran (Technical Grade~ 500K)
Dextran 10 Calibration
Dextran (Technical Grade~ 100K)
Molecular Weignt CRM of Dextran
Dextran 75,000 Mw, Pryogen Free
Dextran 250 Calibration
Dextran 250 Calibration (100 mg)
DEXTRAN AV. MOL. WT. APPROX. 40,000
DextranClinicalGradeMw60,000-90,000
DEXTRAN MOL. WT 5,000,000-40,000,000
DEXTRAN AV. MOL. WT. APPROX. 500,000
Dextran,for biochemistry,low fraction
Dextran,for biochemistry,high fraction
Dextran 40 System Suitability (200 mg)
Dextran 70 System Suitability (200 mg)
DEXTRAN FROM LEUCONOSTOC SSP. MR ~6000
DEXTRAN FROM LEUCONOSTOC MESENTEROIDES
DEXTRAN FROM LEUCONOSTOC SSP. MR ~40000
DEXTRAN FROM LEUCONOSTOC SSP. MR ~70000
Dextran, low fraction, for biochemistry
Dextran from Leuconostoc ssp. Mr ~100000
DEXTRAN FROM LEUCONOSTOC SSP. MR ~500000
Dextran, high fraction, for biochemistry
DEXTRAN STANDARD 5000 CERTIFIED ACC. TO DIN
DEXTRAN STANDARD 1000 CERTIFIED ACC. TO DIN
DEXTRAN STANDARD 12000 CERTIFIED ACC. TO DIN
DEXTRAN STANDARD 50000 CERTIFIED ACC. TO DIN
DEXTRAN STANDARD 80000 CERTIFIED ACC. TO DIN
DEXTRAN STANDARD 25000 CERTIFIED ACC. TO DIN
Dextran, low fraction, for biocheMistry 100GR
Dextran, high fraction, for biocheMistry 100GR
DEXTRAN MOL. WT. APPROX. 500,000*20% SOL UTION
DEXTRAN STANDARD 270000 CERTIFIED ACC. T O DIN
DEXTRAN STANDARD 150000 CERTIFIED ACC. T O DIN
DEXTRAN STANDARD 410000 CERTIFIED ACC. T O DIN
DEXTRAN STANDARD 670000 CERTIFIED ACC. T O DIN
DEXTRAN FROM LEUCONOSTOC SSP. MR ~15000-20000
dextran solution from leuconostoc mesenteroides
DEXTRAN FROM LEUCONOSTOC MESENTEROIDES M R ~60000
DEXTRAN FROM LEUCONOSTOC MESENTEROIDES M R ~200000
DEXTRAN CLINICAL GRADE AV.*MOL. WT. 60,0 00-90,000
DEXTRAN INDUSTRIAL GRADE AV.MOL. WT. 60, 000-90,000
DEXTRAN INDUSTRIAL GRADE AV.MOL. WT. 15, 000-20,000
Dextran (Mw.=5,000,000-40,000,000) (native Dextran)
DEXTRAN INDUSTRIAL GRADE AV. MOL.*WT. 10 0,000-200,0
DEXTRAN STANDARD 270'000 F. LEUCONOSTOC MESENTEROIDES
DEXTRAN STANDARD 1'000 FROM LEUCONOSTOC MESENTEROIDES
DEXTRAN STANDARD 670'000 F. LEUCONOSTOC MESENTEROIDES
DEXTRAN STANDARD 5'000 FROM LEUCONOSTOC MESENTEROIDES
DEXTRAN STANDARD 410'000 F. LEUCONOSTOC MESENTEROIDES
DEXTRAN STANDARD 150'000 F. LEUCONOSTOC MESENTEROIDES
DEXTRAN STANDARD 750'000 F. LEUCONOSTOC MESENTEROIDES*
DEXTRAN STANDARD 12'000 FROM LEUCONOSTOC MESENTEROIDES
DEXTRAN STANDARD 25'000 FROM LEUCONOSTOC MESENTEROIDES
DEXTRAN STANDARD 80'000 FROM LEUCONOSTOC MESENTEROIDES
DEXTRAN STANDARD 50'000 FROM LEUCONOSTOC MESENTEROIDES
DEXTRAN STANDARD 1'400'000 FROM LEUCONO-STOC MESENTEROIDES*
DEXTRAN STANDARD 1'800'000 FROM LEUCONO-STOC MESENTEROIDES*
Dextran from Leuconostoc mesenteroides Vetec(TM) reagent grade
(2R,3S,4R,5R)-2,3,4,5-Tetrahydroxy-6-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]oxan-2-yl]oxyhexanal | [EINECS(EC#)]
232-677-5 | [Molecular Formula]
C17H32O10 | [MDL Number]
MFCD04118900 | [MOL File]
9004-54-0.mol | [Molecular Weight]
396.43 |
| Chemical Properties | Back Directory | [Appearance]
white crystals or powder | [Melting point ]
483 °C (decomp) | [alpha ]
198 º | [density ]
1.00-1.20g/mL | [refractive index ]
185 ° (C=6, H2O) | [storage temp. ]
2-8°C | [solubility ]
H2O: soluble50mg/mL, clear to very slightly hazy, colorless to faintly yellow | [form ]
Solid | [color ]
White to slightly off-white | [PH]
2 - 10 | [Stability:]
Stable. Keep dry. Incompatible with strong oxidizing agents. | [biological source]
bacterial (Leuconostoc mesenteroides) | [optical activity]
+208 to +233 (formaldehyde) | [Water Solubility ]
Soluble in water, dimethyl sulfoxide, ethylene glycol and glycerol. | [Merck ]
2948 | [LogP]
-4.259 (est) | [Uses]
dextran is a polysaccharide with water-binding properties. It is also used to control product viscosity. Some studies indicate a capacity to enhance the anti-aging activity of formulations containing weak acids, as well as to reduce possible skin irritation arising from such acids. | [EPA Substance Registry System]
Dextran(9004-54-0) | [Absorption]
≤0.05 at 375nm in H2O at 10% |
| Hazard Information | Back Directory | [Chemical Properties]
white crystals or powder | [Usage]
exhibit borad spectrum of biological activities, with very low anticoagulant capacity: they activate lipoprotein elimination by lipase stimulation, inhibit cell proliferation in vascular wall, regulate transport of plasmatic proteins and inhibit depositi | [Usage]
also available in pharma grade | [Usage]
antiinflammatory veterinary drug | [Usage]
Dextran is a polysaccharide composed of glucose molecules used as an antithrombotic to reduce blood viscosity and as a volume expander in anemia. Studies show that it inhibits the mannose receptor-med
iated clearance of tissue-type plasminogen activator (t-PA). | [Originator]
LMD 10%,Abbott,US,1967 | [Definition]
dextran: A glutinous glucose polymerproduced by certain bacteria. Itcan be made by fermenting sucrose(cane sugar) and is used as a thickeningagent, as a stabilizer in ice cream,and as a substitute for plasma inblood transfusions. Esters with sulphuricacid yield sodium salts thatare employed as anticoagulant drugs. | [Manufacturing Process]
Sucrose is subjected to the action of the bacterium Leuconosfoc
mesenteroides B 512 and the crude, high-molecular weight dextran thus
formed is hydrolyzed and fractionated to an average molecular weight of
about 40,000 as measured by light-scattering techniques. | [Brand name]
Gentran 40
(Baxter Healthcare). | [Therapeutic Function]
Plasma extender | [General Description]
Dextrans are polysaccharides with molecular weights ≥1,000 Dalton, with a linear backbone of α-linked D-glucopyranosyl repeating units. Dextrans are found as bacterial extracellular polysaccharides. They are synthesized from sucrose by Leuconostoc mesenteroides and Lactobacillus brevis. Bacteria employ dextran in biofilm formation or as a protective coating to evade host phagocytes in the case of pathogenic bacteria.
Dextran from Leuconostoc mesenteroides (Mw: 12,000) may be used as an analytical standard to calibrate the column for gel permeation chromatography (GPC). | [Biochem/physiol Actions]
Dextran is a branched glucan composed of linear a(1→6) linked glucose units and a (1→3) link initiated branches. Dextran ranges in size from 10,000 to 150,000 Kd. Dextrans are used in many applications as volume extenders, stabilizers, matrix components, binding platforms, lubricants and physical structure components. | [Mechanism of action]
| [Veterinary Drugs and Treatments]
Dextran 70 is a relatively low cost colloid for the adjunctive treatment
of hypovolemic
shock. Hetastarch is the more commonly employed
synthetic colloid used today. | [Purification Methods]
Solutions of dextran keep indefinitely at room temperature if 0.2mL of Roccal (10% alkyldimethylbenzylammonium chloride) or 2mg phenyl mercuric acetate are added per 100mL solution. This inhibits mould growth. [Scott & Melvin Anal Biochem 25 1656 1953.] |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
20/21/22-36/37/38 | [Safety Statements ]
24/25-37/39-36-26 | [WGK Germany ]
2
| [RTECS ]
HH9230000
| [F ]
3 | [TSCA ]
Yes | [HS Code ]
39139000 | [Safety Profile]
Suspected carcinogen
with experimental carcinogenic data. When
heated to decomposition it emits acrid
smoke and fumes. See also other
DEXTRANS. | [Toxicity]
LD50 oral in rat: > 3gm/kg |
| Questions And Answer | Back Directory | [Description]
Dextran, a glucose polymer composed predominantly of ol-1,6-glucopyranosidic linkages, is produced from sucrose by Leuconostoc mesenteroides and related organisms and from dextrins by other bacteria.
Dextran is used in a bead form to aid in bioreactor applications, some size-exclusion chromatography matrices and in osmotic stress technique study involved in biological molecules. It can be used as a stabilizing coating to protect metal nanoparticles from oxidation.
Dextrans are long-chain glucose polysaccharides of various relative molecular masses. Dextran 70 (relative molecular mass 70 000) is retained in the intravascular space where, like albumin, it contributes to the colloid oncotic pressure of plasma. Unlike albumin, dextran 70, when given in large amounts, prevents platelet aggregation and facilitates fibrinolysis.
| [Dextran Structure]
Dextran is an α-D-1,6-glucose-linked glucan with side-chains 1-3 linked to the backbone units of the Dextran biopolymer. The degree of branching is approximately 5%. The branches are mostly 1-2 glucose units long.
Dextran can be obtained from fermentation of sucrose-containing media by Leuconostoc mesenteroides B512F.
A fragment of the Dextran structure is illustrated in Figure 1.
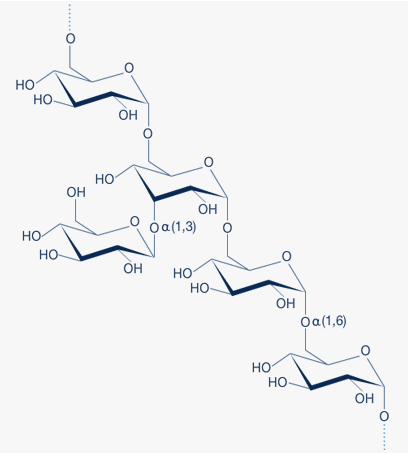
Fig. 1: Dextran is a glucose polymer in which the linkages are predominantly of the α(1,6) type. The degree of α(1,3) branching is generally less than 5% and decreases with decreasing molecular weight
| [History]
In the early 1940's, at the same time as Stacey and his associates in Birmingham were studying bacterial dextrans and Hehre and colleagues in the USA were pursuing the dextran producing activity of cell-free extracts of Leuconostoc , a young Swedish biochemist, B. Ingelman, at the Department of Biochemistry and Physical Chemistry, University of Uppsala began probing the polysaccharides and proteins of sugar beet juice.
One of the critical episodes was the discovery of dextran in an infected sample of the juice. This initiated a series of investigations on the polysaccharide. At the end of 1942, a recently qualified M.D., A. Grönwall, joined the laboratory to study tuberculin. Considerable effort was being devoted at the time to the freeze-drying of blood plasma for military medicine. Within the space of months, Ingelman and Grönwall had stumbled on the idea of using a hydrolyzed dextran as a plasma substitute. After studies on the partial hydrolysis, fractionation, and extensive biological studies, a Swedish pharmaceutical company adopted the project in 1943, and later that year, preliminary clinical trials began. In 1944, under the direction of the surgeon, G. Bohmansson, extensive clinical trials were started at the Regional Hospital in Örebro. The dextran used at that time was derived from Leuconostoc mesenteroides , strain 7E, and was slightly more branched than the present one. By 1947, about four years after the innovation, a 6% solution of a dextran fraction had been approved for clinical use in Sweden and, shortly thereafter, in the U.K., an achievement that would be inconceivable under the present regulatory climate.The product was gradually improved and was designated Dextran 70. Samples of the Swedish product were soon tested clinically in the USA.
Dextran 70 is generally marketed as a 6% solution in normal saline and as such continues to maintain its position worldwide as the plasma volume expander of choice. It is recommended for the treatment of shock or impending shock due, for example, to hemorrhage, burns, surgery or trauma. Dextran 70 also reduces the risk for thrombosis and numerous studies testify to its value in significantly reducing the risk of post-operative fatal pulmonary emboli.
| [Uses]
- The demand for technical dextrans from industry has shown a significant increase in the past decade. Since household sucrose, fruits or fruit beverages could be contaminated with traces of dextrans, ingestion of dextrans, albeit in small amounts, may not be uncommon. Dextran is degraded by dextran-splitting bacteria in the human gut and most of the hydrolysis products can be absorbed to produce a rapid increase in blood sugar and liver glycogen
- However, in the food industry, where innumerable applications of dextrans in foodstuffs were patented in the 50's and 60's, no application appears to have been pursued and the mandatory toxicological studies to gain FDA approval were not performed. Hence in 1977, the GRAS (generally recognized as safe) status of dextrans was deleted. Dextrans are not permitted in the UK or Europe as foodstuff additives, and dextrans do not seem to have been considered by the Joint FAO/WHO Expert Committee on Foodstuff Additives (JECFA). Dextrans are, however, considered as safe as components of food packaging materials.
- Dextran fractions do not appear to be included in the lists of permitted additives (ingredients) for pharmaceutical formulations such as ointments and creams for topical use and tablets and capsules for oral use. However, providing the appropriate documentation is presented, there are no a priori reasons why they may not be used. Indeed several products in which a dextran fraction is used as a non-active ingredient are on the market.
- Purified dextran fractions with high clarity and low chloride levels find extensive applications in the photographic industry. Addition of low concentrations of dextran to the silver emulsion is found to enhance significantly the quality of the images. The effect is presumably attributable to the effect of dextran on the conformation of the gelatin molecules.
- Since Albertsson revealed the enormous potential of 2-phase polymer systems, especially dextran-PEG systems, for the partition of sub-cellular particles and macromolecules, an immense number of applications has evolved. These systems offer a means of fractionation beyond the range of conventional techniques. Some recent applications are: the separation of peripheral blood cells, distinguishing erythrocytes from multiple sclerosis patients, the separation of enzymes, for example pullulanase, from Klebsiella pneumoniae cells, and the partitioning of murine lymphoblasts.
- Dextran has been recommended as a cryoprotective agent for human, animal and plant cells. Thus a mixture of 5% methyl sulphoxide and 9% Dextran 70 was found to afford optimal cryoprotection of human bone marrow committed stem cells.
- The effect of dextrans as adjuvants for prolonging local anesthetic block has been a matter of some debate. Early results had proved somewhat contradictory. Recent reexamination by Hassan and colleagues has revealed that the prolongation of the effect of anesthetic is dependent on the anesthetic used, the MW of the dextran, and the type of dextran derivative used. A prolongation of up to 350% has been obtained.
| [Specification]
Dextran 70 powder Pharmaceutical grade (for injections)
Description
A white, amorphous powder
Identification
To pass test
Clarity and color of solution
To pass test
Chloride
Not more than 0.018 %
Heavy metal
Not more than 20 ppm
Arsenic
Not more than 1.3 ppm
Nitrogen
Not more than 0.010 %
Reducing substances
Not more than 1.0 %
Loss on drying
Not more than 5.0 %
Residue on ignition
Not more than 0.10 %
Intrinsic viscosity (25ºC;)
Whole fraction
0.21 - 0.26 dl/g
7 - 10 % high MW fraction
Not more than 0.35 dl/g
7 - 10 % low MW fraction
Not less than 0.10 dl/g
Antigenicity
To pass test
Storage
Store at the temperature below 25 ºC. Protect from light and moisture.
| [Diverse Applications]
- Dextran has traditionally been used in infusion fluid and volume expander products. However, dextran has a very diverse application area ranging from vaccines over ophthalmic use to stabilizer of biological components. Some of the uses in life sciences.
- Vaccines: Dextran can be part of vaccines as a carrier, a back-bone and/or as a stabilizer of the antigen or other subunits. Derivatives of dextran in particular DEAE-dextran are often also used.
- Eye Drops or Similar Solutions: Dextran is often used in eye drops or similar solution for ophthalmic application. Dextran can be used as the active ingredient in eye drops due to its lubricating nature or as tear-replacement. This application is used to relieve dry, irritated eyes. Common causes for dry eyes include wind, sun, heating/air conditioning, computer use/reading, and certain medications. Furthermore dextran can also be used in eye drops with a medicating component.
- Protein Stabilization: The dextran molecule is known to benefit on structural stability of freeze-dried products, protein stability and the recovery of enzyme activity after freeze-drying.
- Excipient in Lyophilization (freeze-drying): Lyophilization is a commonly used technique for formulation development of small molecules, proteins and vaccines which are unstable in aqueous medium and/or are thermolabile in nature. Lyophilization of drug alone, however, presents certain formulation development challenges, which may be overcome by incorporation of excipients in the formulation. Dextran is often used as excipient during lyophilization as a bulking agent and/or a collapse temperature modifier.
- Cryo-Protectant: Dextran can be used as a cryo-protectant in combination with DSMO, glycerol etc. Dextrans can be used to cryo-preserve cell lines, stem cell preparations and biological samples in general. It is believed these cryo-protectants contributes to a more controlled formation of ice crystals which leads to lesser damage to cell membranes and organelles.
- Storing Organs for Transplantation: Dextran is widely accepted as solution for storing and preparing organs for transplantation. Traditionally Corneas has been stored and/or prepared for transplantation dextran, but now a multitude of different organs and tissues are being stored in dextran solution for increased longevity or dextran are used in preparation prior to transplantation.
- Oral Products: Dextran has a wide use in oral pharmaceuticals. Dextran is applied for solidity and consistency of substance during processing e.g. freeze drying. Dextran can also be used to alter the dissolution profile of drug formulations.
- Blood Cell Separation: Dextran can be applied in the reversible aggregation of human red blood cells, yet the mechanistic details governing the process are still being explored. The process is useful for separation of red blood cells from other cells and components of the blood.
- Blood Volume Expander: Intravenous solutions with dextran function both as volume expanders and means of parenteral nutrition. Such a solution provides an osmotically neutral fluid that once in the body is digested by cells into glucose and free water.
Pharmacosmos Pharmaceutical Quality Dextran 1
| [Production Methods]
Dextran for clinical and technical products is produced in most developed countries throughout the world. In the West, most producers use the Leuconostoc mesenteroides NRRL B-512(F) or B-512 strain for the fermentation. In other parts of the world, alternative strains appear to be used.
Most major producers of dextran employ a process based on the batchwise culture of Leuconostoc in the presence of sucrose. The viscous culture fluid is then precipitated in ethanol or methanol, whereafter the native dextran obtained is hydrolyzed in dilute acid and the desired dextran is isolated by fractionation. Although the present state of the art offers alternative methods of producing defined fractions, most producers are still operating a procedure introduced about 35 years ago. In introducing any change, a producer must be convinced that, not only must the new process be more efficient in man-power and materials, but the final product must conform in every respect with the medical requirements for safety and efficacy.
The organism, Leuconostoc mesenteroides NRRL B-512(F), is a member of the Lactobacillaceae family, genus Leuconostoc and species mesenteroides (134). The organism produces spherical or ovoid cells and classifies as a gram-positive facultative anaerobe. Apart from dextran and lactic acid, it produces, inter alia , carbon dioxide, ethanol, mannitol and acetic acid.
| [Dextran Safety]
Dextran has been used in numerous products for human use for decades
Products include both IV, IM, oral, and topical administration. These products have typically been used globally including US and Europe. Examples include Dextran use for plasma volume expansion, in numerous eyes drops, and oral products like Spasfon and Opalmon.
For decades Dextran has had the Generally Recognized As Safe (GRAS) label from the US FDA, which was renewed in 2013. (Link to article).
The established use of dextran through decades of human use in numerous products with different administration forms clearly establishes the low risk profile of Dextran.
This is well known to competent authorities around the world, and it is taken into consideration when the regulatory authorities are establishing their toxicological, preclinical and clinical requirements.
|
|
|