| Identification | Back Directory | [Name]
P-TOLUENESULFINIC ACID SODIUM SALT TETRAHYDRATE | [CAS]
868858-48-4 | [Synonyms]
4-TOLUENESULFINIC ACID NA 4H2O
SodiuM p-Toluenesulfinate
SODIUM P-TOLUENESULFINATE TETRAHYDRATE
SODIUM 4-TOLUENESULFINATE TETRAHYDRATE
Sodium p-Toluenesulfinate Tetrahydrate
P-TOLUENESULFINIC ACID SODIUM SALT TETRAHYDRATE
4-TOLUENESULFINIC ACID, SODIUM SALT, TETRAHYDRATE | [EINECS(EC#)]
212-538-5 | [Molecular Formula]
C7H15NaO6S | [MDL Number]
MFCD00149644 | [MOL File]
868858-48-4.mol | [Molecular Weight]
250.25 |
| Chemical Properties | Back Directory | [storage temp. ]
Inert atmosphere,Room Temperature | [Water Solubility ]
Soluble in water | [form ]
powder to crystal | [color ]
White to Light yellow | [Merck ]
14,9532 | [CAS DataBase Reference]
868858-48-4 |
| Hazard Information | Back Directory | [Uses]
Sodium p-toluenesulfinate Hydrate is used in organic chemical reactions as an alkylating agent, non-oxidizing catalyst, intermediate for the synthesis of biologically active compounds. of grades are available including Mil Spec (military grade), ACS, Reagent and Technical Grade, Food, Agricultural and Pharmaceutical Grade, Optical Grade, USP and EP/BP (European Pharmacopoeia/British Pharmacopoeia). | [Synthesis]
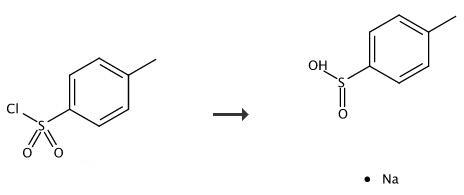
The aryl sulfonyl chloride (10.0 mmol, 1.0 equiv) was dissolved in 30 mL water. Sodium sulfite (16.0 mmol, 1.6 equiv) and sodium bicarbonate (16.0 mmol, 1.6 equiv) were added, and the reaction mixture was refluxed for 3 h. The water was evaporated, and ethanol was added to the residue. The suspension was heated for 10 min, cooled, and filtered through a 20 μm polyethylene frit. This was repeated twice with the residue from the filtration. The ethanol fractions were combined, and the solvent was evaporated under vacuum, The sodium arylsulfinates were isolated as white powders. |
|
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
|