| Identification | Back Directory | [Name]
3-(4-Nitro-1-oxo-1,3-dihydroisoindol-2-yl)piperidine-2,6-dione | [CAS]
827026-45-9 | [Synonyms]
6-Piperidinedione
4-Nitro LenalidoMide
Lenalidomide Impurity B
Lenalidomide EP Impurity B
Lenalidomide 4-Nitro Impurity
3-(4-Nitro-1-oxo-1, 3-dihydro-isoindol-
3-dihydro-4-nitro-1-oxo-2H-isoindol-2-yl)-
3-(4-Nitro-1-oxoisoindolin-2-yl)piperidin-2,6-dione
3-(4-nitro-1-oxoisoindolin-2-yl)piperidine-2,6-dione
3-(4-nitro-1-oxo-2-isoindolinyl)piperidine-2,6-dione
3-(7-nitro-3-oxo-1H-isoindol-2-yl)piperidine-2,6-dione
3-(4-Nitro-1-oxo-1,3-dihydroisoindol-2-yl)piperidine-2
3-(1-keto-4-nitro-isoindolin-2-yl)piperidine-2,6-quinone
3-(4-Nitro-1-oxo-1,3-dihydroisoindol-2-yl)piperidine-2,6-dione
3-(4-nitro-1-oxo-2,3-dihydro-1H-isoindol-2-yl)piperidine-2,6-dione
3-(1,3-Dihydro-4-nitro-1-oxo-2H-isoindol-2-yl)-2,6-piperidinedione
3-(4-Nitro-1,3-dihydro-1-oxo-2H-isoindol-2-yl)-2,6-piperidinedione
2,6-Piperidinedione, 3-(1,3-dihydro-4-nitro-1-oxo-2H-isoindol-2-yl)-
(3S)-3-(4-Nitro-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione | [EINECS(EC#)]
1592732-453-0 | [Molecular Formula]
C13H11N3O5 | [MDL Number]
MFCD18064658 | [MOL File]
827026-45-9.mol | [Molecular Weight]
289.24 |
| Chemical Properties | Back Directory | [Melting point ]
>250°C (dec.) | [Boiling point ]
616.6±55.0 °C(Predicted) | [density ]
1.546±0.06 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,2-8°C | [solubility ]
DMSO (Slightly, Heated), Methanol (Slightly) | [form ]
Solid | [pka]
10.55±0.40(Predicted) | [color ]
Beige to Brown | [InChI]
InChI=1S/C13H11N3O5/c17-11-5-4-10(12(18)14-11)15-6-8-7(13(15)19)2-1-3-9(8)16(20)21/h1-3,10H,4-6H2,(H,14,17,18) | [InChIKey]
JKPJLYIGKKDZDT-UHFFFAOYSA-N | [SMILES]
N1C(=O)CCC(N2CC3=C(C2=O)C=CC=C3[N+]([O-])=O)C1=O |
| Hazard Information | Back Directory | [Chemical Properties]
White crystalline | [Uses]
Lenalidomide (L328000) analog. Lenalidomide impurity. | [Preparation]
3-aminopiperidine-2,6-dione hydrochloride (III) (25 g, 0.15 mol) and dimethyl sulfoxide (150 mL) were charged into 500 mL 3N RBF. Triethylamine (62 g, 0.61 mol) was added slowly to the above reaction mixture under nitrogen over 10 min. Methyl 2-(bromomethyl)-3-nitrobenzoate (II) (45.8 g, 0.16 mol) of example 3 dissolved in dimethyl sulfoxide (50 mL) was added to the reaction mixture under nitrogen over 20 min and heated to 50-55 °C. 3-(4-Nitro-1-oxo-1,3-dihydroisoindol-2-yl)piperidine-2,6-dione was obtained by purification.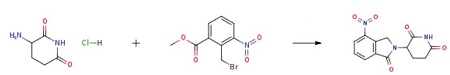 |
|
|