| Identification | Back Directory | [Name]
(R)-(-)-3,3'-BIS(TRIPHENYLSILYL)-1,1'-BINAPHTHYL-2,2'-DIYL HYDROGENPHOSPHATE, 95% | [CAS]
791616-55-2 | [Synonyms]
Carreira SALDIPAC Ligand
(S)-3,3μ-Bis(triphenylsilyl)-1,1μ-binaphthyl-2,2μ-diyl hydrogenphosphate
(R)-(-)-3,3'-Bis(triphenylsilyl)-1,1'-binaphthyl-2,2'-diyl hydrogenphosphate 96%
(R)-(-)-3,3'-BIS(TRIPHENYLSILYL)-1,1'-BINAPHTHYL-2,2'-DIYL HYDROGENPHOSPHATE, 95%
(R)-3,3'-Bis(triphenylsilyl)-1,1'-binaphthyl-2,2'-diyl
Hydrogen Phosphate,99%e.e.
2,6-bis(triphenylsilyl)dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-ol 4-oxide
(11bS)-4-hydroxy-2,6-bis(triphenylsilyl)dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepine 4-oxide
(11bR)-4-Hydroxy-2,6-bis(triphenylsilyl)-4-oxide-dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin
(11bR)-2,6-Bis(triphenylsilyl)-4-hydroxy-4-oxide-dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin
MacMillan TiPSY catalyst, (11bR)-4-Hydroxy-2,6-bis(triphenylsilyl)dinaphtho-[2,1-d:1μ,2μf]-1,3,2-dioxaphosphepin 4-oxide
MacMillan TiPSY catalyst, (11bS)-4-Hydroxy-2,6-bis(triphenylsilyl)dinaphtho-[2,1-d1μ,2μf]-[1,3,2]-dioxaphosphepin 4-oxide | [Molecular Formula]
C56H41O4PSi2 | [MDL Number]
MFCD09265079 | [MOL File]
791616-55-2.mol | [Molecular Weight]
865.082 |
| Chemical Properties | Back Directory | [Melting point ]
329-335 °C | [alpha ]
-197° (c=1, CHCl3) | [density ]
1.32±0.1 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [form ]
Powder | [pka]
1.17±0.20(Predicted) | [color ]
white to light-yellow | [optical activity]
[α]22/D -197°, c = 1 in chloroform |
| Questions And Answer | Back Directory | [Reaction]
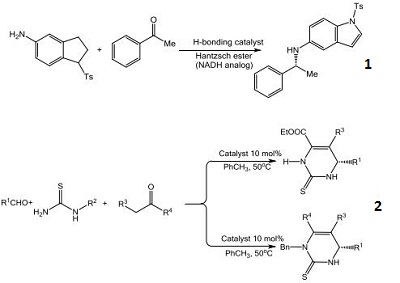
- A chiral phosphoric acid catalyst providing a convenient strategy for the enantioselective construction of protected primary amines and a highly stereoselective method for the reductive amination of heterocyclic amines.
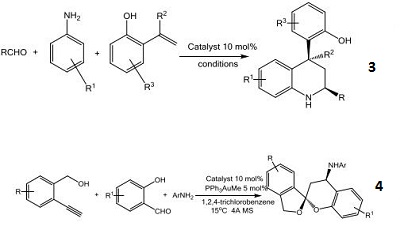
- Chiral phosphoric acid used for the enantioselective Biginelli and Biginelli-like reactions.
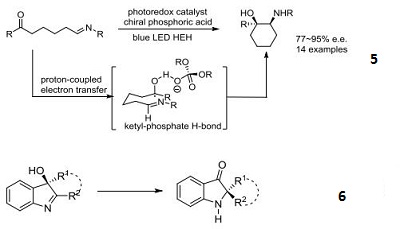
- Chiral phosphoric acid organocatalyst used in the asymmetric, three-component Povarov reaction involving 2-hydroxystyrenes. An efficient method to access structurally diverse cis-disubstituted tetrahydroquinolines in high stereoselectivities of up to >99:1 dr and 97% ee.
- A gold/chiral phosphoric acid catalyst used for the highly stereoselective, three-component reaction of salicylaldehydes, anilines, and alkynols to give aromatic spiroacetals in high yields and stereoselectivities.
- The first highly enantioselective catalytic protocol for the reductive coupling of ketones and hydrozones.
- Reagent-controlled regioselectivity enabled by dual activation.
|
|
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
| Company Name: |
Sigma-Aldrich
|
| Tel: |
021-61415566 800-8193336 |
| Website: |
https://www.sigmaaldrich.cn |
|