| Identification | Back Directory | [Name]
2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)Methyl)benzoic acid | [CAS]
763114-26-7 | [Synonyms]
EOS-61877
Olapali impurity 25
Olaparib Intermediate 1
Olaparib intermediate 763114-26-7
2-Fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)
2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)Methyl)
2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)Methyl)be
2-Fluoro-5-(4-oxo-3,4-dihydrophthalazin-1-ylm ethyl)benzo
2-fluoro-5-[(4-oxo-3H-phthalazin-1-yl)Methyl]benzoic acid
2-Fluoro-5-(4-oxo-3,4-dihydrophthalazin-1-ylmethyl)benzoic a...
2-Fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoicaci
2-Fluoro-5-[(4-oxo-3,4-dihydrophthalazin-yl)methyl] benzoic acid
-Fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)benzoic acid
2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)Methyl)benzoic acid
2-Fluoro-5-[(4-oxo-3,4-dihydro-1-phthalazinyl)methyl]benzoic Acid
5-[(3,4-Dihydro-4-oxo-1-phthalazinyl)methyl]-2-fluoro-Benzoic acid
Benzoic acid, 5-[(3,4-dihydro-4-oxo-1-phthalazinyl)methyl]-2-fluoro-
(2-Fluoro-5-(4-oxo-3,4-dihydrophthalazin-1-ylMethyl)benzoic acid)763114-26-7
C16H11FN2O3 2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)Methyl)benzoic acid 763114-26-7 | [EINECS(EC#)]
800-110-6 | [Molecular Formula]
C16H11FN2O3 | [MDL Number]
MFCD14636678 | [MOL File]
763114-26-7.mol | [Molecular Weight]
298.269 |
| Chemical Properties | Back Directory | [density ]
1.42 | [storage temp. ]
Sealed in dry,Room Temperature | [form ]
powder | [pka]
3.19±0.10(Predicted) | [color ]
White to off-white | [InChI]
InChI=1S/C16H11FN2O3/c17-13-6-5-9(7-12(13)16(21)22)8-14-10-3-1-2-4-11(10)15(20)19-18-14/h1-7H,8H2,(H,19,20)(H,21,22) | [InChIKey]
PAXLJNGPFJEKQX-UHFFFAOYSA-N | [SMILES]
C(O)(=O)C1=CC(CC2C3=C(C=CC=C3)C(=O)NN=2)=CC=C1F |
| Hazard Information | Back Directory | [Application]
2-Fluoro-5-(4-oxo-3,4-dihydrophthalazin-1-ylmethyl)benzoic Acid is an intermediate in the synthesis of olaparib. Olaparib is a PARP polymerase inhibitor. | [Synthesis]
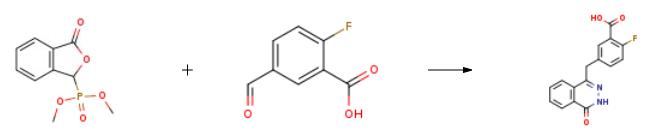
Compound 3 (2.6g, 0.011mol)And 2-fluoro-5-formylbenzoic acid (2.1g, 0.012mol)Dissolved in anhydrous tetrahydrofuran (25ml), cooled to 0 ,Slowly add triethylamine (1.0ml, 0.007mol) dropwise, then warm up to room temperature for 5h, then slowly raise the temperature to 70 , add hydrazine hydrate (5.1ml, 0.107mol) for 3h, and drop to room temperature , Add appropriate amount of hydrochloric acid (2mol / L) to adjust the pH to acidic, no longer precipitate solids. Filter with suction, wash the product with water and ethyl acetate, and dry to obtain a yellow solid (1.9g, yield 83%) |
|
|