| Identification | Back Directory | [Name]
NORTRIPTYLINE | [CAS]
72-69-5 | [Synonyms]
Ateben
C07274
Avantyl
Aventyl
lambeck
Lumbeck
Sesaval
Noritren
allegron
Psychostyl
Nortrityline
NORTRIPTYLINE
Nortryptiline
nortryptyline
Desitriptilina
Noramitriptyline
Demethylamitriptylene
Demethylamitriptyline
demethylamitryptylene
demethyl-amitryptylin
Demethylamitryptyline
Desmethylamitriptyline
NORTRIPTYLINE USP/EP/BP
Amitryptyline, demethyl-
Amitriptyline EP Impurity C
5-[3-(Methylamino)propylidene]dibenzo[a,E]cyclohepta[1,5]diene
5-(3-(methylamino)propylidene)dibenzo(a,e)cyclohepta(1,5)diene
5-(alpha-methylaminopropylidene)dibenzo[a,d]cyclohepta[1,4]diene
10,11-dihydro-5-(3-methylaminopropylidene)dibenzo(a,d)cycloheptene
5-(3-Methylaminopropylidene)-10,11-dihydro-5H-dibenzo(a,d)cycloheptene
3-(10,11-dihydro-5h-dibenzo[a,d]cyclohepten-5-ylidene)-n-methylpropylamine
10,11-Dihydro-N-methyl-5H-dibenzo(a,d)cycloheptane-delta,gamma-propylamine
10,11-dihydro-5-(3-methylaminopropylidene)-5h-dibenzo[a,d][1,4]cycloheptene
10,11-dihydro-n-methyl-5h-dibenzo(a,d)cycloheptene-delta(5,gamma)-propylamin
10,11-dihydro-n-methyl-5h-dibenzo[a,d]cycloheptene-delta(5,gamma)-propylamine
3-(10,11-Dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-N-methyl-1-propanamine
5h-dibenzo(a,d)cycloheptene-delta(5,gamma)-propylamine,10,11-dihydro-n-methyl
5H-Dibenzo[a,d]cycloheptene-delta5,gamma-propylamine, 10,11-dihydro-N-methyl-
3-(10,11-dihydro-5h-dibenzo[a,d]-cyclohepten-5-ylidene)-n-methyl-1-propanamine
1-propanamine,3-(10,11-dihydro-5h-dibenzo(a,d)cycloheptene-5-ylidene)-n-methyl
1-Propanamine, 3-(10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-N-methyl-
N-[3-(10,11-DIHYDRO-5H-DIBENZO[A,D][7]ANNULEN-5-YLIDENE)PROPYL]-N-METHYLAMINE HYDROCHLORIDE | [EINECS(EC#)]
200-788-8 | [Molecular Formula]
C19H21N | [MDL Number]
MFCD00242775 | [MOL File]
72-69-5.mol | [Molecular Weight]
263.38 |
| Chemical Properties | Back Directory | [Melting point ]
58 °C | [Boiling point ]
396.62°C (rough estimate) | [density ]
0.9790 (rough estimate) | [refractive index ]
1.4900 (estimate) | [storage temp. ]
4°C, protect from light | [form ]
Solid | [pka]
pKa 9.7 (Uncertain) | [color ]
White to off-white | [BCS Class]
1 | [EPA Substance Registry System]
Nortriptyline (72-69-5) |
| Hazard Information | Back Directory | [Originator]
Aventyl,Lilly,UK,1963 | [Uses]
Antidepressant. | [Uses]
Nortriptyline is a drug with a relatively short latent period of action. It is
practically devoid of sedative effects. It is used in manic-depressive psychoses, in all forms
of endogenous depression, and also in major depressive conditions. | [Definition]
ChEBI: An organic tricyclic compound that is 10,11-dihydro-5H-dibenzo[a,d][7]annulene substituted by a 3-(methylamino)propylidene group at position 5. It is an active metabolite of amitriptyline. | [Manufacturing Process]
A mixture of 114.5 g of 5-(3-chloropropylidene)dibenzo[a,d]cyclohepta[1,4] diene, 75 ml of benzene, and about 400 ml of methylamine is heated in an autoclave at 120°C for six hours. The excess methylamine is distilled from the reaction mixture under vacuum and the residue is stirred with 300 ml of water. Acidification of the mixture with hydrochloric acid causes the separation of the hydrochloride of 5-(3-methylaminopropylidene)dibenzo[a,d]
cyclohepta[1,4]diene. The product is collected by filtration and is purified by recrystallization from a mixture of absolute ethanol and ethyl acetate. MP 210°C to 212°C.
| [Brand name]
Aventyl Hydrochloride (Lilly); Aventyl Hydrochloride (Ranbaxy); Pamelor (Tyco). | [Therapeutic Function]
Antidepressant | [Clinical Use]
Tricyclic antidepressant | [Safety Profile]
Poison by ingestion,intraperitoneal, and intravenous routes. When heated todecomposition it emits toxic fumes of NOx. | [Synthesis]
Nortriptyline is 5-(3-methylaminopropyliden)-10,11-dihydrodibenzcyclo�heptene (7.1.17). Nortriptyline differs from desipramine in the same manner in which amitriptyline differs from imipramine. In nortriptyline, the nitrogen atom in the central part of the tricyclic system of desipramine is replaced by a carbon atom, which is bound to a side chain by a double bond.
Two suggested methods of nortriptyline synthesis are based on the N-demethylation of amitriptyline. The third way utilizes the reaction of methylamine with 5-(3-bromopropyli�den)-10,11-dihydro-5H-dibenz[a,d]cycloheptene (7.1.18).
According to the first scheme, demethylation takes place by the reaction of amitripty�line (7.1.4) with methyliodide, which leads to the formation of a quaternary ammonium salt (7.1.16), the reaction of which with methylamine at a relatively high temperature gives the desired nortriptyline (7.1.17) [25].

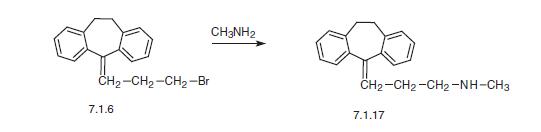
According to the third scheme, nortriptyline is synthesized by reacting methylamine with 5-(3-bromopropyliden)-10,11-dihydro-5H-dibenz[a,d]cycloheptene (7.1.6) [8].
| [Drug interactions]
Potentially hazardous interactions with other drugs
Alcohol: increased sedative effect.
Analgesics: increased risk of CNS toxicity with
tramadol; possibly increased risk of side effects with
nefopam; possibly increased sedative effects with opioids.
Anti-arrhythmics: increased risk of ventricular
arrhythmias with amiodarone - avoid; increased
risk of ventricular arrhythmias with disopyramide,
flecainide or propafenone; avoid with dronedarone.
Antibacterials: increased risk of ventricular
arrhythmias with delamanid, moxifloxacin and
possibly telithromycin - avoid with moxifloxacin.
Anticoagulants: may alter anticoagulant effect of
coumarins.
Antidepressants: enhanced CNS excitation and
hypertension with MAOIs and moclobemide -
avoid; concentration possibly increased with SSRIs;
risk of ventricular arrhythmias with citalopram and
escitalopram - avoid; increased risk of convulsions
with vortioxetine.
Antiepileptics: convulsive threshold lowered;
concentration reduced by carbamazepine,
fosphenytoin, phenobarbital and possibly phenytoin.
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol.
Antipsychotics: increased risk of ventricular
arrhythmias especially with droperidol, haloperidol,
pimozide, risperidone and sulpiride - avoid;
increased antimuscarinic effects with clozapine
and phenothiazines; concentration increased by
antipsychotics.
Antivirals: increased risk of ventricular arrhythmias
with saquinavir - avoid; concentration possibly
increased with ritonavir.
Atomoxetine: increased risk of ventricular
arrhythmias and possibly convulsions.
Beta-blockers: increased risk of ventricular
arrhythmias with sotalol.
Clonidine: tricyclics antagonise hypotensive effect;
increased risk of hypertension on clonidine withdrawal.
Dapoxetine: possible increased risk of serotonergic
effects - avoid.
Dopaminergics: avoid use with entacapone; CNS
toxicity reported with selegiline and rasagiline.
Pentamidine: increased risk of ventricular
arrhythmias.
Sympathomimetics: increased risk of hypertension
and arrhythmias with adrenaline and noradrenaline;
metabolism possibly inhibited by methylphenidate. | [Metabolism]
Nortriptyline is a secondary amine dibenzocycloheptene TCA as well as the major metabolite of
amitriptyline. Similar to desipramine, nortriptyline appears in mother's milk and is metabolized by CYP2D6 to
the primary amine and by ring hydroxylation to its E-10-hydroxy metabolite. Approximately
one-third of a dose of nortriptyline is excreted in urine as metabolites within 24 hours, and small amounts are
excreted in feces via biliary elimination. | [storage]
4°C, protect from light |
|
| Company Name: |
AKASH PHARMA EXPORTS
|
| Tel: |
+91-9388123451 +91-9846039283 |
| Website: |
www.UNDERCONSTRUCTION |
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
|