| Identification | Back Directory | [Name]
1-Bromodibenzothiophene | [CAS]
65642-94-6 | [Synonyms]
1-Bromodibenzothiophene
Dibenzothiophene, 1-bromo-
1-bromodibenzo[b,d]thiophene
1-Bromodibenzothiophene ISO 9001:2015 REACH | [Molecular Formula]
C12H7BrS | [MOL File]
65642-94-6.mol | [Molecular Weight]
263.15 |
| Hazard Information | Back Directory | [Uses]
1-Bromodibenzothiophene can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development processes and chemical production processes. | [Synthesis]
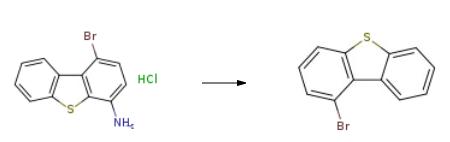
Into a 1 L four-necked round bottom flask equipped with a stirrer, a Liebig condenser (not required), a 100 mL dropping funnel and a thermometer, 43.5 g (0.156 mol) of the amine hydrochloride obtained in the above ), 240 mL of acetic acid, 55 mL of concentrated hydrochloric acid and 143 mL of water were placed and cooled to -5 ?? C. or lower in an ice water bath. Next, an aqueous solution of 12.0 g (0.173 mol) of sodium nitrite dissolved in 67 mL of water was added dropwise from the dropping funnel at a temperature not exceeding 5 ?? C., and the mixture was stirred at 5 ?? C. or less for 1 hour.The resulting diazo solution was transferred to a 1 L dropping funnel and set in a 1 L 4-necked round bottom flask equipped with a stirrer, a Liebig condenser (not required) and a thermometer, and then 50 % Hypophosphorous acid and 90 mL of water were added, and the mixture was cooled to -5 ?? C. or lower in an ice water bath. Subsequently, the diazo solution was added dropwise at a temperature not exceeding 5 ?? C., after stirring for 1 hour in an ice water bath, it was returned to room temperature and stirred at room temperature for 48 hours.The obtained reaction solution was extracted twice with 310 mL of ethyl acetate, then washed twice with 310 mL of water, dried with magnesium sulfate, magnesium sulfate was removed by suction filtration, and the solvent was distilled off under reduced pressure. Subsequently, the obtained crude oil was purified on a silica gel column using n-heptane as a developing solution to obtain 33.8 g (yield: 82.5%) of the desired bromide. |
|
|