| Identification | Back Directory | [Name]
1,1-Dioxo-hexahydro-1l6-thiopyran-4-carboxylic acid | [CAS]
64096-87-3 | [Synonyms]
1,1-Dioxo-tetrahydrothiop...
1,1-Dioxo-1lambda(6)-thiane-4-carboxylicacid
1,1-Dioxohexahydrothiopyran-4-carboxylic acid
1,1-Dioxo-tetrahydrothiopyran-4-carboxylic acid
1-Dioxo-hexahydro-1l6-thiopyran-4-carboxylic acid
Tetrahydrothiopyran-4-carboxylic acid 1,1-dioxide
1,1-Dioxo-hexahydro-1l6-thiopyran-4-carboxylic acid
tetrahydro-thiopyrane-4-carboxylic acid 1,1-dioxide
1,1-dioxo-hexahydro-1λ6-thiopyran-4-carboxylic acid
2H-Thiopyran-4-carboxylic acid, tetrahydro-, 1,1-dioxide | [EINECS(EC#)]
815-471-5 | [Molecular Formula]
C6H10O4S | [MDL Number]
MFCD03102802 | [MOL File]
64096-87-3.mol | [Molecular Weight]
178.21 |
| Chemical Properties | Back Directory | [Melting point ]
195-196℃ (acetone ligroine ) | [Boiling point ]
456.6±38.0 °C(Predicted) | [density ]
1.422±0.06 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Room Temperature | [form ]
powder | [pka]
4.05±0.20(Predicted) | [color ]
White to off-white |
| Hazard Information | Back Directory | [Synthesis]
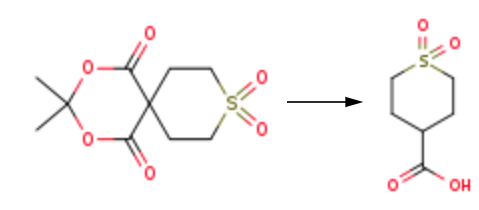
3,3-Dimethyl- 2, 4-dioxa-9-thiaspiro[5.5]undecane-1,5-dione-9, 9-dioxide 1 (20.0 g, 76.3 mmol) and then 6 N hydrochloric acid (60 mL) and Antifoam 204 (200 mg, 1 wt % relative to 1) were charged to a 250-mL, three-neck round-bottom flask, equipped with a mechanical stirrer and a thermocouple. The reaction was then heated to reflux, and the distillate was collected until the internal temperature reached 100 eC; after that, the reaction was allowed to reflux for an additional 8 hours or until LC/MS showed a complete conversion of 1 to 2. The reaction was then cooled to room temperature and held for ~12 h when the product crystallized from the solution. The slurry was cooled to 0 °C for 1 h and then filtered. The cake was dried in a vacuum oven at 60 eC, with nitrogen bleed, overnight to give the product as a white, crystalline solid 1,1-Dioxo-tetrahydrothiopyran-4-carboxylic acid (8.84 g, 49.6 mmol, 65% yield.
|
|
|