| Identification | Back Directory | [Name]
1H-indole-3-propylamine | [CAS]
6245-89-2 | [Synonyms]
Einecs 228-361-1
1H-indole-3-propylamine
1H-Indole-3-propanamine
N-propyl-1H-indol-3-amine
1H-Indole-3-propan-1-amine
(1H-Indol-3-yl)-1-propanamine | [EINECS(EC#)]
228-361-1 | [Molecular Formula]
C11H14N2 | [MDL Number]
MFCD00130194 | [MOL File]
6245-89-2.mol | [Molecular Weight]
174.24 |
| Chemical Properties | Back Directory | [Melting point ]
64 °C | [Boiling point ]
353.7±25.0 °C(Predicted) | [density ]
1.125±0.06 g/cm3(Predicted) | [storage temp. ]
under inert gas (nitrogen or Argon) at 2–8 °C | [pka]
17.29±0.30(Predicted) |
| Questions And Answer | Back Directory | [Synthesis]
A solution of LAH (1.0 M in ether, 36.5 mL, 36.5 mmol) was cooled to 0 °C, and a solution of the cyanoethyl indole (2.78 g, 16.3 mmol) was added slowly. Then, the solution was heated at reflflux for 3 h. It was cooled to 0 °C and quenched by dropwise addition of water (20 mL) followed by 1 N sodium hydroxide (40 mL). The phases were separated, and the aqueous phase was extracted with ether. The combined organic phases were washed with brine and then dried (potassium hydroxide). Evaporation of the solvent gave 2.6 g (92%) of homotryptamine as a yellow oil, which solidifified on standing. The hydrochloride was prepared by dissolving the amine in a minimum of ethanol and then a saturated solution of hydrogen chloride in ether was added until no additional salt formation was observed. The hydrochloride was recrystallized from ethanol.
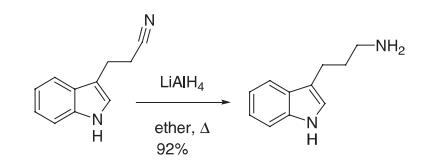
Reference: Kuehne, M. E.; Cowen, S. D.; Xu, F.; Borman, L. S. J. Org. Chem. 2001, 66, 5303–5316. |
|
| Company Name: |
SynAsst Chemical.
|
| Tel: |
021-60343070 |
| Website: |
www.approvedhomemanagement.com/ShowSupplierProductsList15848/0.htm |
| Company Name: |
Cool Pharm, Ltd
|
| Tel: |
021-58581007 18019463053 |
| Website: |
http://www.coolpharm.com |
|