| Identification | Back Directory | [Name]
TRIMETHYLTIN HYDROXIDE | [CAS]
56-24-6 | [Synonyms]
trimethylstannanol
hydroxytrimethyltin
trimethylhydroxytin
hydroxytrimethyl-ti
rimethyltin,hydrate
trimethyl-tihydroxide
TRIMETHYLTIN HYDROXIDE
TIN TRIMETHYL HYDROXIDE
Hydroxytrimethyltin(IV)
Trimethylstannyl alcohol
hydroxytrimethyl-stannan
hydroxytrimethylstannane
trimethylstannylhydroxide
Trimethyltinhydroxide,98%
STANNANE,HYDROXYTRIMETHYL-
TRIMETHYLTIN(IV) HYDROXIDE
Stannane, hydroxytrimethyl- (8CI,9CI)
TriMethyltin hydroxide, 98% (CH3)3SnOH
Trimethyltin hydroxide, 98% (CH3)3SnOH F.W.180.80 | [Molecular Formula]
C3H10OSn | [MDL Number]
MFCD00013929 | [MOL File]
56-24-6.mol | [Molecular Weight]
180.82 |
| Chemical Properties | Back Directory | [Melting point ]
118°C | [Boiling point ]
80°C | [RTECS ]
WH8400000 | [Fp ]
26℃ | [solubility ]
Chloroform (Soluble, Sonicated), Methanol (Slightly, Sonicated), Water (Sparingly) | [form ]
Powder | [pka]
6.36±0.70(Predicted) | [color ]
White | [Water Solubility ]
Soluble in water. | [Sensitive ]
Moisture Sensitive | [Exposure limits]
ACGIH: TWA 0.1 mg/m3; STEL 0.2 mg/m3 (Skin)
NIOSH: IDLH 25 mg/m3; TWA 0.1 mg/m3 | [EPA Substance Registry System]
Stannane, hydroxytrimethyl- (56-24-6) |
| Hazard Information | Back Directory | [Chemical Properties]
Colorless, white crystals. Soluble in water and many organic solvents. | [Uses]
Drug. | [Reactions]
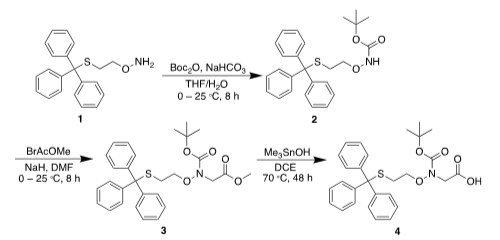
The synthesis of N-Boc-N-(2-(tritylthio)ethoxy)glycine (4) is illustrated in Scheme 2. O-(2-(tritylthio)ethyl)hydroxylamine (1) was synthesized using the literature method. The aminooxy group was then protected with Boc, followed by alkylation with methyl bromoacetate in the presence of sodium hydride. Saponification of the methyl ester with trimethyltin hydroxide afforded N-Boc-N-(2-(tritylthio)ethoxy)glycine (4) in an overall yield of 19% after three steps of reactions. The Boc and Trt protecting groups in building block 4 are stable to Fmoc SPPS and are readily removable by treatment with trifluoroacetic acid (TFA).
 | [Hazard]
A poison. | [References]
[1] Anderson, Kirsty M., et al. "Trimethyltin hydroxide: a crystallographic and high Z′ curiosity." Crystal Growth & Design, 2011, 11 3: 820-826. DOI:10.1021/cg101464j.
[2] K. C. NICOLAOU PROF. DR. A Mild and Selective Method for the Hydrolysis of Esters with Trimethyltin Hydroxide?[J]. Angewandte Chemie International Edition, 2005, 44 9: 1378-1382. DOI:10.1002/anie.200462207.
[3] ROBIN K HARRIS Patrick R Kenneth J Packer. High-resolution Tin-119 NMR of solid trimethyltin hydroxide[J]. Journal of Magnetic Resonance (1969), 1985, 61 3: Pages 564-566. DOI:10.1016/0022-2364(85)90199-4. |
|
| Company Name: |
Domole Scientific Gold
|
| Tel: |
13275595566 13275595566 |
| Website: |
www.domole.com/ |
| Company Name: |
INTATRADE GmbH
|
| Tel: |
+49 3493/605464 |
| Website: |
www.intatrade.de |
| Company Name: |
Alfa Aesar
|
| Tel: |
400-6106006 |
| Website: |
http://chemicals.thermofisher.cn |
| Company Name: |
Energy Chemical
|
| Tel: |
021-021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
|