| Identification | Back Directory | [Name]
9,10[1',2']-Benzenoanthracene, 2,3,6,7,14,15-hexabromo-9,10-dihydro- | [CAS]
55805-81-7 | [Synonyms]
JACS-55805-81-7
2,3,6,7,14,15-hexabromotriptycene
2����,3,6����,7��,12���,13-Hexabromotriptycene
2,3,6,7,14,15-Hexabromo-9,10-dihydro-9,10-[1,2]benzenoanthracene
9,10[1',2']-Benzenoanthracene, 2,3,6,7,14,15-hexabromo-9,10-dihydro- | [Molecular Formula]
C20H8Br6 | [MDL Number]
MFCD31700802 | [MOL File]
55805-81-7.mol | [Molecular Weight]
727.71 |
| Hazard Information | Back Directory | [Uses]
2,3,6,7,14,15-Hexabromo-9,10-dihydro-9,10-[1,2]benzenoanthracene can be used as impurity standard and reference substance, mainly used in laboratory research and development process and chemical production process middle. | [Synthesis]
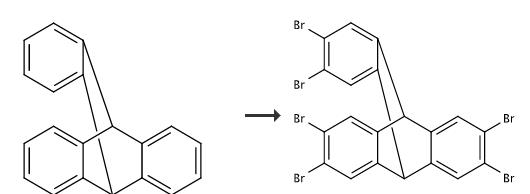
Triptycene (1.06 g, 4.18 mmol) was dissolved in chloroform (80 mL) in a round-bottom flask. Iron filings (30 mg) were added, and the solution was stirred at 25 °C. Bromine (1.35 mL, 26.3 mmol) was added, and the solution was refluxed for 1 h, during which time the initially reddish-brown solution turned reddish-orange. The flask was removed from heat, and chloroform and excess bromine were removed under vacuum. The resulting brown powder was dissolved in chloroform (100 mL) and flushed through a pad of silica using additional chloroform as eluent (100 mL). The filtrate was evaporated to dryness. The crude white powder (2.83 g, 98%) was crystallized from acetone yielding C20H8Br6 · (acetone)2 (0.88 g, 29%), mp >350 °C. The mother liquor was evaporated and the residue was crystallized from acetone to afford a second crop of crystals (0.97 g, C20H8Br6 · (acetone)2, 32%). The combined yield was 1.85 g, 61%: 2-H yield 2.83 g, 98% |
|
| Company Name: |
Henan Alfachem Co.,Ltd. Gold
|
| Tel: |
0371-0371-55051623 18137891487 |
| Website: |
http://www.approvedhomemanagement.com/supplier/14555231/ |
|